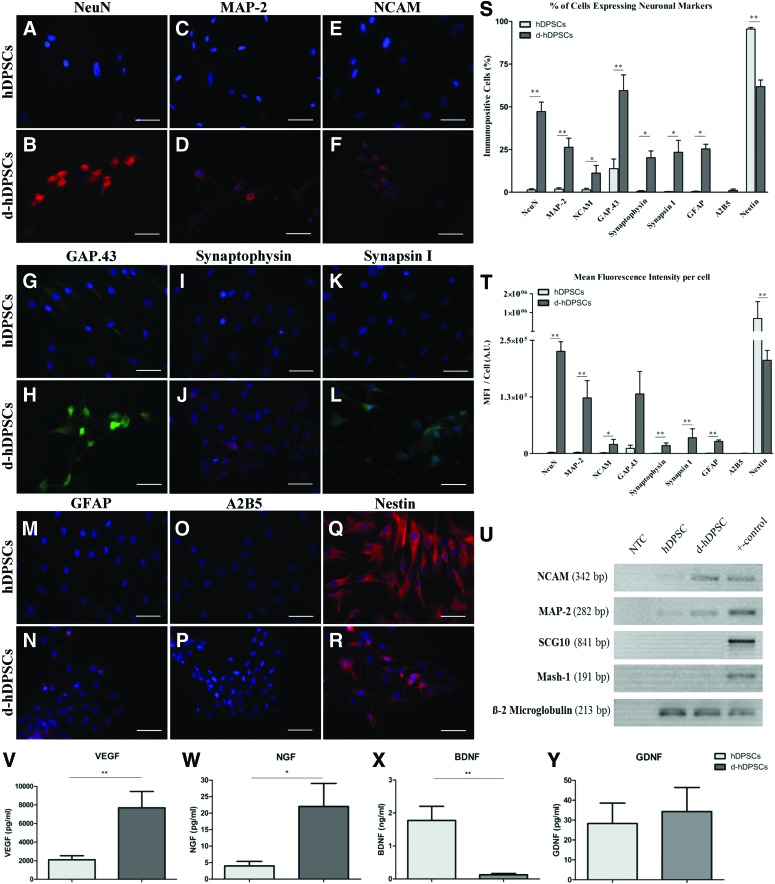
FIG. 3.
Differentiated hDPSCs (d-hDPSCs) acquired immune reactivity for neuron-related markers and showed an increased expression of neuronal genes in addition to an altered secretome. Compared with controls, neurogenic maturation induced the expression of neuronal nuclei [NeuN; (A, B)], microtubule-associated protein 2 [MAP-2; (C, D)], neural cell adhesion molecule [NCAM; (E, F)], growth-associated protein 43 [GAP.43 (G, H)], synaptophysin (I, J), synapsin I (K, L), and glial fibrillary acid protein [GFAP; (M, N)]. A2B5 expression was low (<2%) in both hDPSCs and d-hDPSCs (O, P). Nestin expression was decreased in d-hDPSCs compared with controls (Q, R). The presented images in (B, D, F, H, J, L, N, and R) are representative for the immune-positive cells in d-hDPSC cultures (n=5). Quantitatively, both the mean fluorescence intensity (MFI) per cell and the percentage of cells expressing neuronal markers were significantly increased in d-hDPSCs, except for nestin, which was significantly decreased following neurogenic differentiation (S, T). No significant difference in the MFI per cell was detected for GAP.43. Reverse transcriptase–polymerase chain reaction analysis confirmed the upregulation of MAP-2 and NCAM on the gene expression level (n=4). The expression of the neuron-related genes, Mash-1 and SCG10, was not observed (U). β-2 Microglobulin was used as an endogenous control. Vascular endothelial growth factor (VEGF, n=6) and nerve growth factor (NGF, n=5) secretion was significantly increased in d-hDPSCs compared with hDPSCs (V, W). Brain-derived neurotrophic factor (BDNF, n=6) secretion was significantly decreased after neurogenic maturation (X). Differential secretion of glial cell-derived neurotrophic factor (GDNF, n=5) between hDPSCs and d-hDPSCs could not be observed (Y). A.U., arbitrary units; NTC, nontemplate control. Scale bars (A–R)=50 μm; *P≤0.05; **P≤0.01; data are presented as mean±standard error of the mean. Color images available online at www.liebertpub.com/scd