Figure 2.
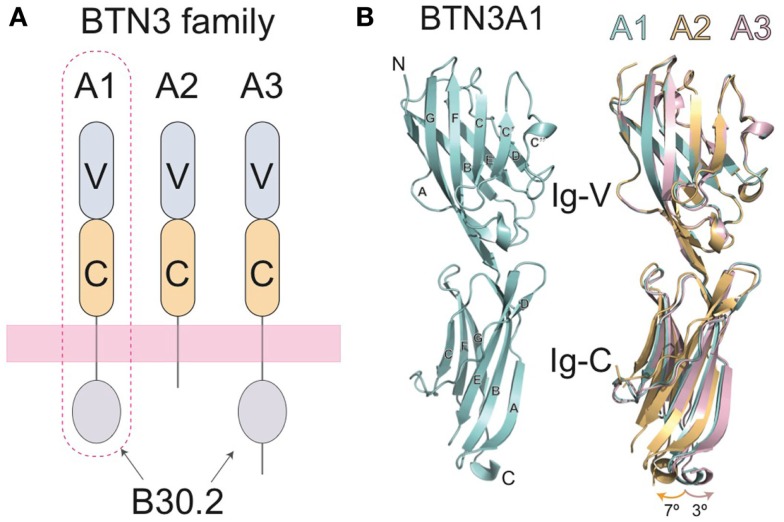
Domain organization of the butryophilin-3 (BTN3) proteins. (A) The BTN3A extracellular domains are members of the B7-superfamily with a membrane-proximal IgC domain and an N-terminal IgV domain. The extracellular domains between the three isoforms are highly similar, but are linked, via a single-pass transmembrane region, to intracellular domains that vary amongst the three BTN3A family members. BTN3A1 and BTN3A3 both contain intracellular B30.2 domains whereas BTN3A2 does not. BTN3A1, circled with a dotted line, has been shown to be necessary for pAg-induced activation of Vγ9Vδ2 T cells. (B) The three-dimensional structures of the extracellular domain of BTN3A1 is shown in cyan (left) and superimposed with the A2 and A3 isoforms (right) shown in gold and pink, respectively. The structures are highly homologous, with only small variations in the hinge angles between the IgV and Ig-C domains.