Figure 2.
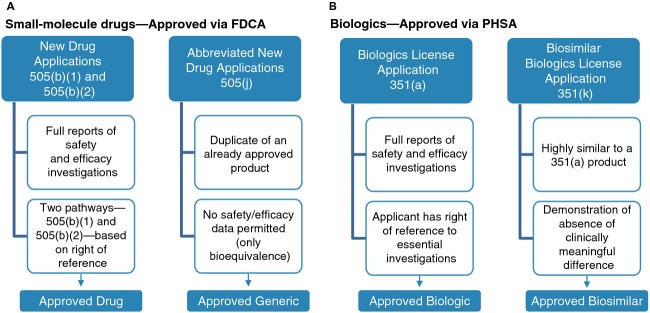
Approval pathways for (A) small-molecule drugs versus generics and (B) biologics versus biosimilars. New small-molecule drugs are approved under a New Drug Application as authorized by the Food, Drug, and Cosmetic Act (FDCA). A subsequent generic drug can be approved via an Abbreviated New Drug Application that demonstrates that it is a duplicate of the small-molecule reference drug (i.e., same active ingredient, strength, dosage form, route of administration, and conditions of use; bioequivalent). In contrast, new biologics are evaluated and approved under a 351(a) Biologics License Application as authorized by the Public Health Service Act (PHSA). The Biologic Price Competition and Innovation Act of 2009 created a 351(a) biosimilar Biologics License Application pathway that requires demonstration that the biosimilar is highly similar to its biologic reference product, notwithstanding minor differences in clinically inactive components, and that it has no clinically meaningful differences from its reference product in terms of safety, purity, and potency 10.
