Figure 2.
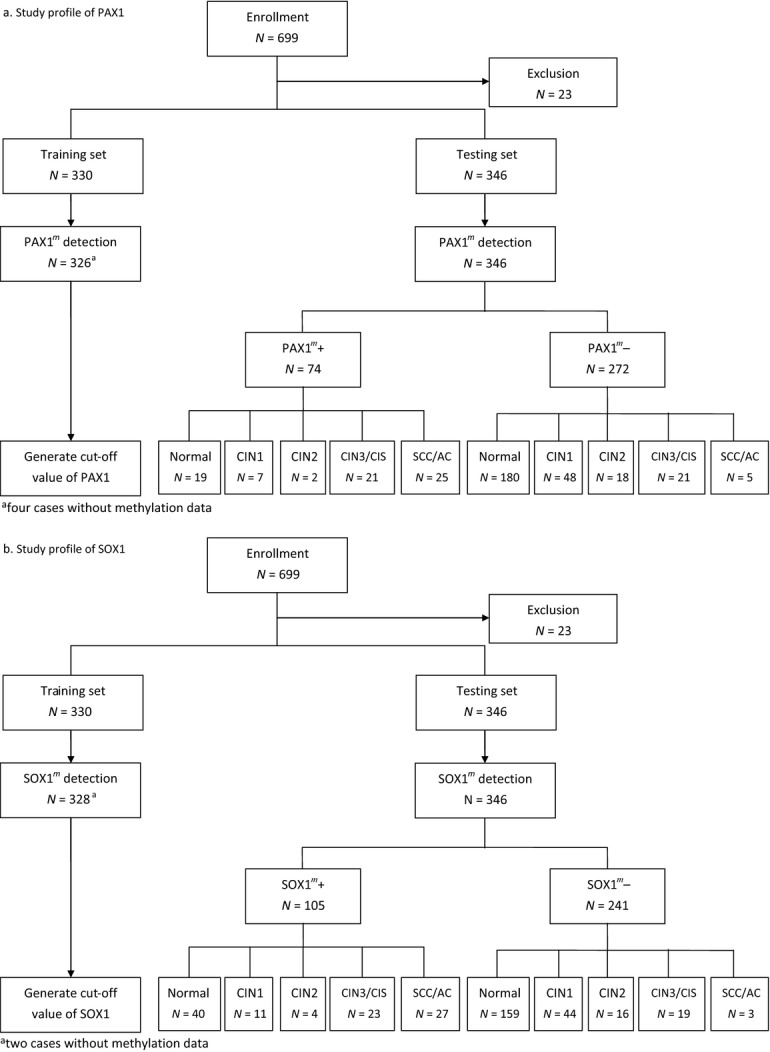
Enrollment and outcome. Women with known cytology results were invited to undergo a HPV DNA test and DNA methylation test within 2 months of the Pap smear screening. All women with abnormal cytology underwent colposcopy and biopsy. Histopathology diagnoses were used as endpoints for the analysis except for women with normal cytology. Twenty-three women were excluded when checking the inclusion criteria. Key: Normal, normal cervical cytology without biopsy; CIN1, cervical intraepithelial neoplasia type 1; CIN2, cervical intraepithelial neoplasia type 2; CIN3, cervical intraepithelial neoplasia type 3; CIS, carcinoma in situ; SCC, squamous cell carcinoma; AC, adenocarcinoma.
