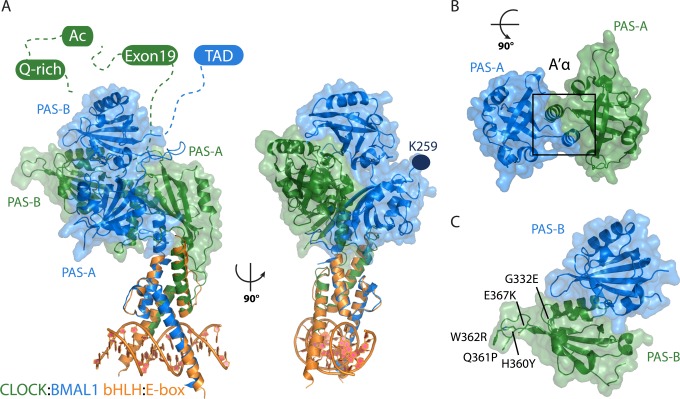
Figure 8.
CLOCK:BMAL1 bHLH-PAS dimer structure. (A) Crystal structures of the bHLH dimer:E-box complex (orange, PDB entry 4H10) and the bHLH-PAS-AB dimer (PDB entry 4F3L) overlaid with CLOCK (green) and BMAL1 (blue). Dashed lines indicate intrinsically disordered C-terminal regions containing functionally important regions, including CLOCK exon 19, the acetyl-CoA-binding motif (Ac), the glutamine-rich (Q-rich) region, and the BMAL1 transcriptional activation domain (TAD). K259 (dark blue) is the SUMOylation site on BMAL1 PAS-A. (B) Depiction of interactions between the PAS-A domains, shown in isolation at a 90° rotation relative to the left structure in panel A. (C) Depiction of interactions between the PAS-B domains, shown in isolation, with mutations within the CLOCK PAS-B domain that decrease the level of CRY binding and/or repression of the CLOCK:BMAL1 complex.