Figure 3.
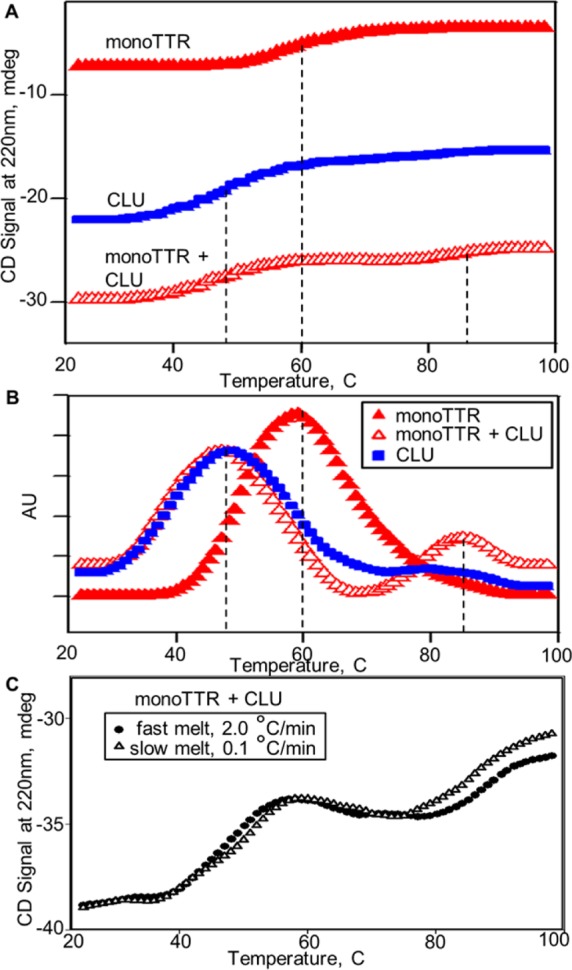
Effect of clusterin on thermal unfolding of transthyretin (TTR) monomers. (A) Thermal unfolding profiles for monoTTR, CLU, and an equimolar mixture of the proteins were constructed from CD data recorded at 220 nm with samples heated continuously at a rate of 2 °C/min. The apparent melting temperatures (Tm) for samples, indicated by vertical dashed lines, were determined from peak maxima obtained from first-derivative calculations (B) of the melting data. CLU alone (blue squares) and the monoTTR/CLU mixture (open red triangles) exhibit similar lower-temperature structural transitions (Tm values of 48 and 47 °C, respectively) and a plateau at ∼60 °C. MonoTTR alone (filled red triangles) exhibits a single structural transition with a Tm of 60 °C and a plateau at ∼80 °C. The monoTTR/CLU mixture also displays a second unique, higher-temperature structural transition (>80 °C). (C) Comparison of monoTTR/CLU mixture unfolding curves recorded at fast (2 °C/min) and slow (0.1 °C/min) heating rates, showing a rate-dependent shift in the high-temperature (>80 °C) melting transition.
