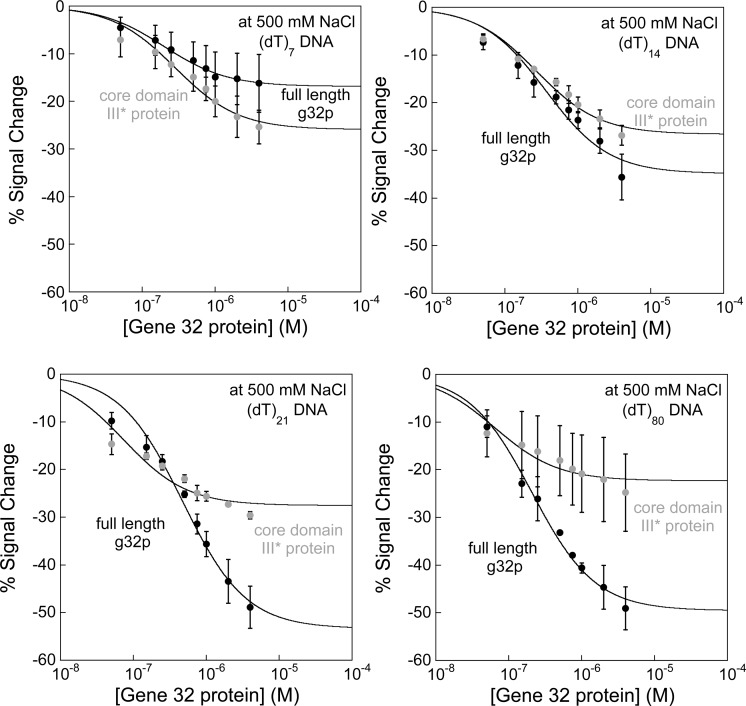
Figure 5.
Oligo(dT) E-DNA sensors distinguish between cooperative binding of full-length g32p and noncooperative binding of the core domain (*III) fragment. Sensors modified with (dT)7, (dT)14, (dT)21, and (dT)80 DNA probes are utilized to differentiate between cooperative and noncooperative binding, with the full-length g32p and truncated core domain (*III) protein used as targets. At high ionic strength, the full-length g32p cooperatively binds single-strand DNA in a probe-length-dependent manner (black lines), while the *III protein binds tightly and noncooperatively to DNA (gray lines). As seen in the binding curves, the full-length g32p generally exhibits weaker DNA–protein binding interactions relative to *III protein, which is characteristic of cooperativity between protein molecules. Except in the plots showing binding to (dT)7, which is fit to the Langmuir expression, lines are drawn to guide the reader’s eye.