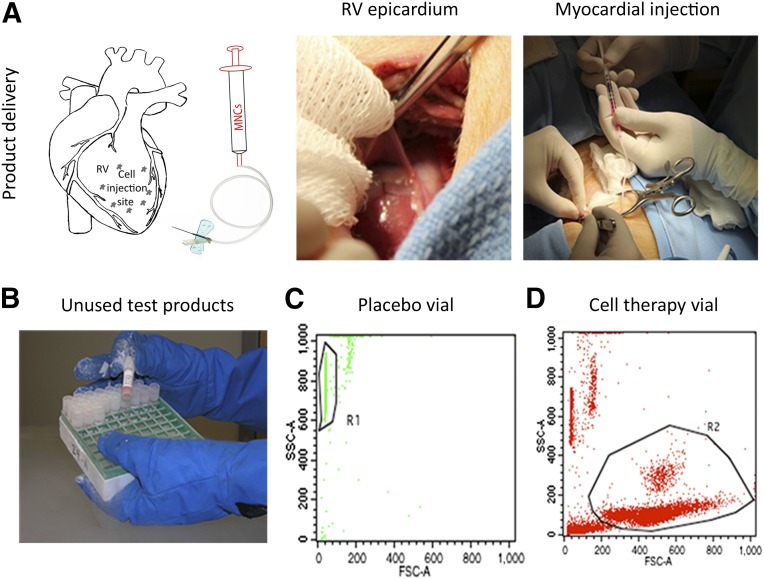
Figure 3.
Autologous umbilical cord blood-derived mononuclear cells versus placebo transplantation and quality assurance testing of unused frozen products. (A): Scheme of the injection target area. Avoiding major blood vessels and the conduction system, a 2–4-cm diameter target area was identified on the surface of the right ventricle. Epicardial injections of cell product and placebo were delivered to 5–15 sites calculated as 0.1 ml per site with a total dose of 0.1 ml/kg of body weight (left). The pericardium was reflected to expose the epicardial surface of the right ventricle through a lateral thoracotomy at T4–T5 intercostal space (middle). The cell/placebo injection into the porcine right ventricle myocardium in a 4-week-old piglet was carried out using a 1-ml syringe connected to the delivery device with a 27-gauge needle on flexible tubing over 20 seconds (right). (B): Frozen cells and placebo vials that were not used for myocardial injection after randomization were kept in liquid nitrogen for quality assurance purposes. (C): Flowchart showing lack of cells from an unused vial labeled as placebo. R1 gate shows beads from Trucount tubes (BD Biosciences, Franklin Lakes, NJ, http://www.bdbiosciences.com) used for counting the absolute number of cells. (D): Flowchart showing presence of leukocytes by FSC/SSC (R2 gate) after a vial containing umbilical cord blood mononuclear cells was analyzed to confirm its content vial. Abbreviations: FSC, forward scatter; MNCs, mononuclear cells; RV, right ventricle; SSC, side scatter.