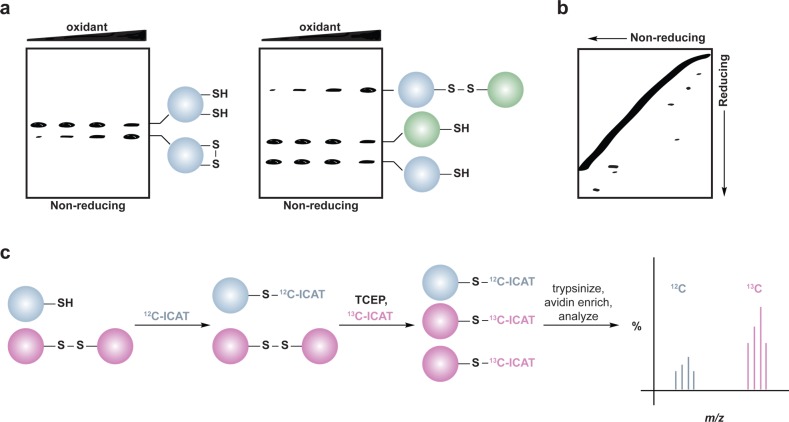
Figure 7.
Methods for detection and identification of protein disulfides. (a) Differential migration of proteins containing intra- and intermolecular disulfide bonds. Samples are resolved under nonreducing SDS-PAGE conditions. Intramolecular disulfides can facilitate enhanced protein migration in some proteins as compared to the reduced species (left). Intermolecular disulfide complexes migrate at the combined molecular weight of the individual proteins (right). (b) Redox 2D-PAGE. Protein samples are first separated by nonreducing gel electrophoresis to separate disulfide-bonded complexes by size (top). The proteins are subsequently reduced in-gel with DTT, alkylated with NEM or IAM, and separated in the second dimension under reducing conditions (down). Proteins that are not involved in intermolecular disulfide complexes run at the diagonal. Proteins involved in disulfide complexes migrate off the diagonal and can be identified by in-gel digestion and LC-MS/MS (not shown). (c) OxICAT method combines the ICAT technology with differential alkylation of reduced and oxidized thiols to permit quantification of oxidized residues. Cell lysates are generated in the presence of trichloroacetic acid and detergents to facilitate exposure of all protein cysteines while inhibiting thiol/disulfide exchange. Reduced thiols (blue) are subsequently blocked with the light (12C) ICAT reagent (blue), oxidized proteins (purple) are reduced with TCEP, and nascent thiols are alkylated with the heavy (13C) ICAT reagent (purple). Samples are trypsinized and labeled peptides are avidin enriched. Eluted peptides are analyzed by LC-MS and heavy and light ICAT-labeled peptides are chemically identical, but differ in mass by 9 Da. The percentage of a particular thiol that is oxidized in a sample is determined by the ratio of heavy (13C) to light (12C) signal intensity from the corresponding peptide. While TCEP can reduce all reversible oxoforms (e.g., disulfides, sulfenic acid, S-nitrosothiols), sulfenic acids and S-nitrosothiols are often acid-labile and likely lost during sample preparation. As such, OxICAT is likely most suitable to detect cysteines involved in disulfide bonds.