Figure 7.
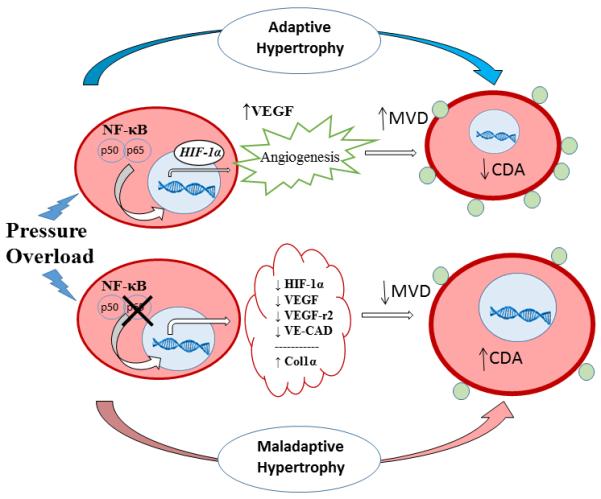
Working model. In a normal cardiomyocyte, hypertrophic stress promotes p65 NF-κB translocation to the nucleus and binding of its target transcription factors, including HIF1α. HIF1α initiates angiogenic pathways including upregulation of VEGF that increases microvascular density (MVD) and decreases capillary domain area (CDA), thereby allowing for adequate nutrient/oxygen delivery and adaptive physiologic response. In the absence of p65 NF-κB, the cardiomyocyte is unable to enhance HIF1α expression and the angiogenic response is blunted (decreased MVD, and increased CDA). In addition to baseline increased collagen 1α synthesis, the p65-deleted cardiomyocyte outgrows its vascular supply, thereby more rapidly progressing to a maladaptive state.
