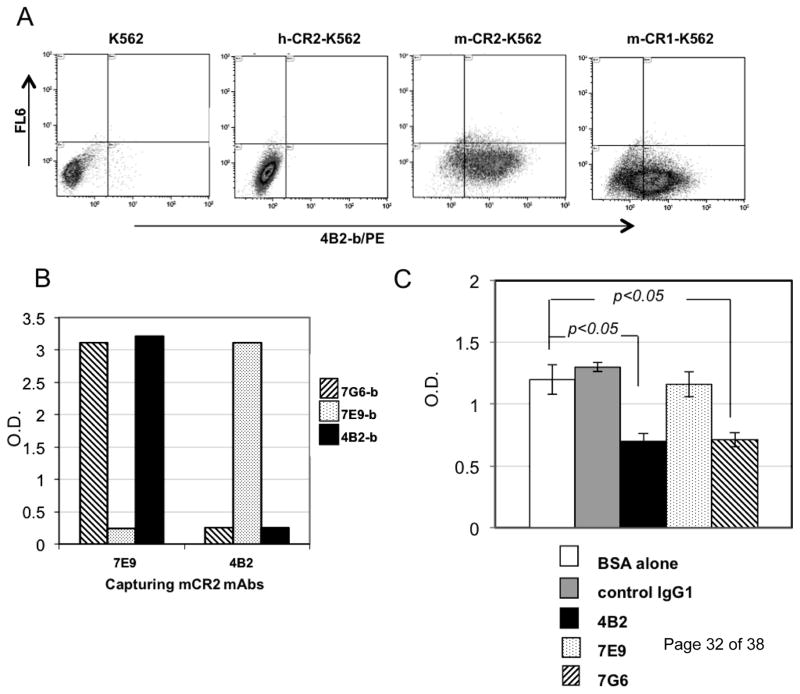
Fig. 1. mAb 4B2 specifically binds to mouse CR2 and CR1 and blocks binding of C3d to CR2.
(A) K562 cells transfected with whole length human CR2 (h-CR2), mouse CR2 (m-CR2) and mouse CR1 (m-CR1) proteins were analyzed by flow cytometry for binding by mAb 4B2. (B) Cross competition analysis wherein mAb 4B2 was assessed by ELISA for its ability to block binding of the previously described inhibitory rat anti-mouse CR2/CR1 mAb 7G6 to CR2 (SCR1–4). For the study, mouse CR2 (SCR1–4) protein was captured by either the non-cross-inhibitory rat anti-mouse CR2/CR1 mAb7E9 or mAb 4B2, and was detected by using either biotinylated mAb 7G6-b (striped), mAb 7E9-b (dotted), or mAb 4B2-b (black). Capturing mouse CR2 with mAb 7E9 did not affect binding of either mAbs 7G6 or 4B2 to recombinant protein. However, when mouse CR2 was captured by mAb 4B2, only biotinylated mAb 7E9 was able to detect the recombinant protein, and the mAb 7G6 epitope was blocked by 4B2 binding. (C) ELISA plate was coated with mouse CR2(SCR1–4) protein and incubated with BSA alone (unfilled) or purified mAbs: control IgG1 (gray), 7E9 (dotted), 7G6 (striped), and 4B2 (black). Plates were washed and biotinylated human C3dg that had been cross-linked by streptavidin labeled with horseradish peroxidase (C3dg-b/ST/HRP) was added. mAb 4B2 blocked the interaction between mouse CR2 and C3dg similarly to mouse CR2 blocking rat mAb 7G6.. * p<0.05.