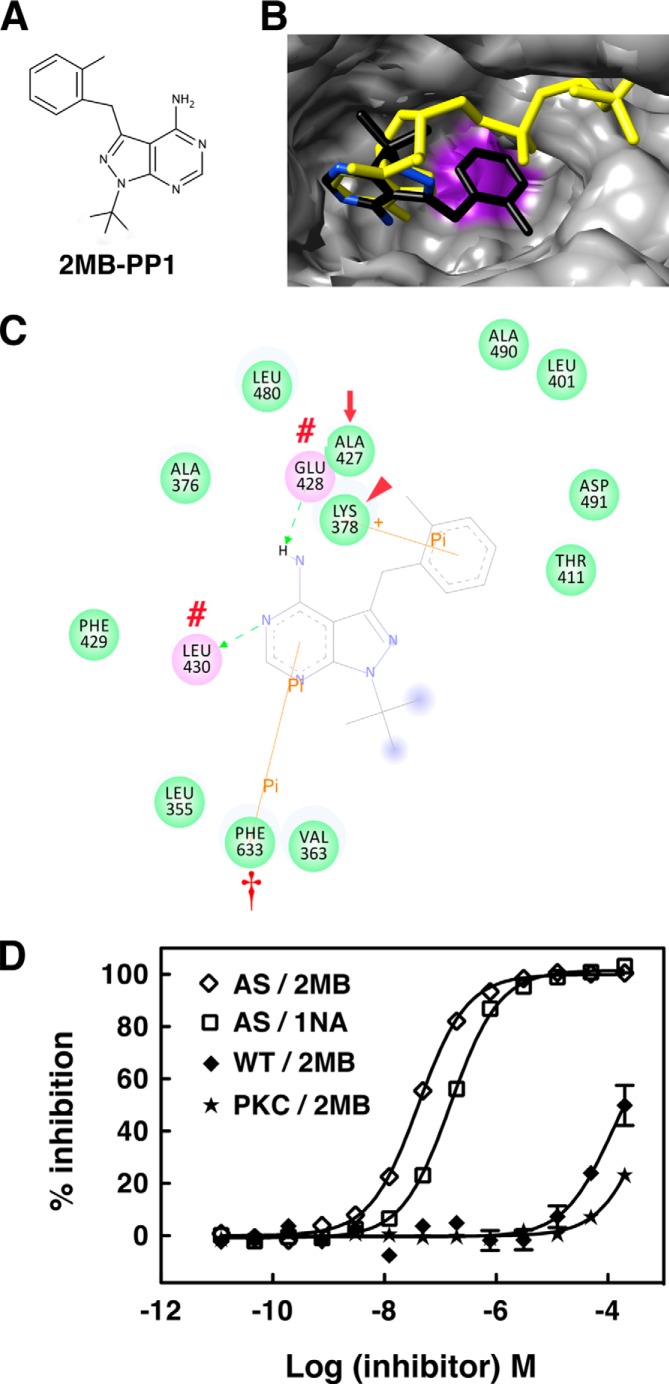
FIGURE 12.
AS PKCδ is more sensitive to inhibition by 2MB-PP1 than 1NA-PP1. A, chemical structure of 2MB-PP1. B and C, interactions of 2MB-PP1 in the nucleotide-binding pocket of AS PKCδ. B, shown are the superimposed structure of 2MB-PP1 (colored by atom type) and ATP (yellow) in AS PKCδ. Ala-427 is highlighted in purple in AS PKCδ. C, 2MB-PP1-interacting residues in the nucleotide-binding pocket of AS PKCδ. 2MB-PP1 forms hydrogen bonds with Glu-428 and Leu-430 (red number symbols), π-σ interaction with Lys-378 (red arrowhead), and π-π interaction with Phe-633 (red dagger). The gatekeeper is indicated by a red arrow. D, inhibition of AS PKCδ, WT PKCδ, and a commercial mixture of PKC isozymes (PKC) (Calbiochem) by 2MB-PP1 and 1NA-PP1 was measured using fluorescence polarization assays (n = 3). Error bars, S.E.