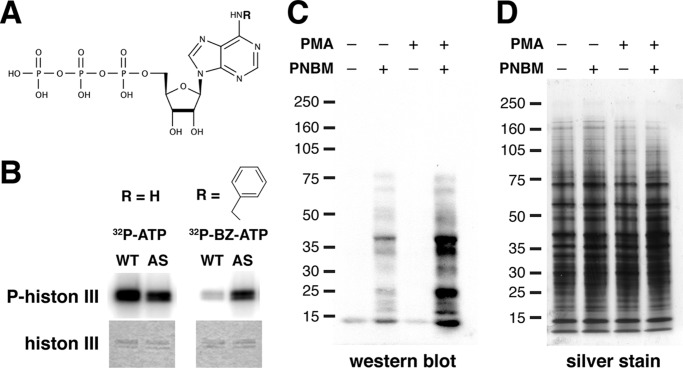
FIGURE 2.
AS PKCδ, but not WT PKCδ, utilized N6-(benzyl)-ATP (BZ-ATP) to phosphorylate substrates. A, chemical structure of an ATP analog. R, site of N6 modification. B, phosphorylation of histone 3 by WT and AS PKCδ in the presence of [γ-32P]ATP or N6-(benzyl)-[γ-32P]ATP. Top, autoradiogram of phosphorylated histone 3; bottom, Coomassie-stained histone 3. C, mouse neutrophil lysates (100 μg) were labeled with AS PKCδ and N6-(benzyl)-ATPγS. After para-nitrobenzyl mesylate (PNBM) alkylation, the labeled substrates were detected by Western blot analysis using an antibody that detects thiophosphate esters. Immunoreactivity was increased in samples treated with the PKC activator PMA. D, the same amounts of neutrophil lysates as in D were loaded and visualized by silver staining.