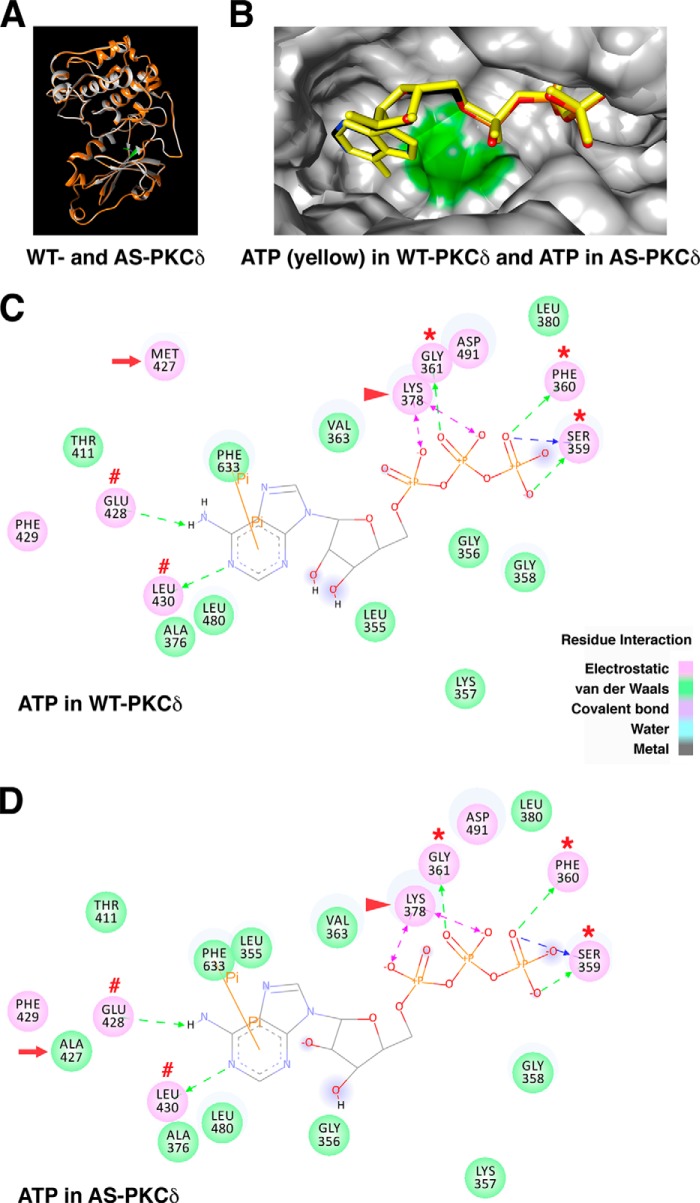
FIGURE 7.
Comparison of ATP interactions in the nucleotide-binding pocket of WT and AS PKCδ. A, the homology model of WT PKCδ (gray) was superimposed with the AS PKCδ model (orange). The gatekeeper residue Met-427 in WT PKCδ is highlighted in green. B, ATP (yellow) docked in WT PKCδ was superimposed with ATP (colored by atom type: nitrogen (blue), carbon (black), oxygen (red), and phosphorus (orange)) docked in AS PKCδ. Shown are the ATP-interacting residues in the nucleotide-binding pocket of WT (C) and AS PKCδ (D). Residues involved in electrostatic (hydrogen bond, charge, or polar), van der Waals, covalent bond, water, and metal interactions with the ligand are shaded in pink, green, magenta, aquamarine, and dark gray, respectively. Hydrogen bond interactions with amino acid main chain and amino acid side chain residues are represented by green and blue dashed arrows directed toward the electron donor. Charged interaction is shown by a pink dashed arrow with heads on both sides. Pi interaction is represented by an orange line with, π indicating the interaction. In both WT and AS PKCδ, ATP forms hydrogen bonds with Glu-428 and Leu-430 (red number symbols) near the gatekeeper (red arrow) and Ser-359, Phe-360, and Gly-361 in the glycine-rich loop (red asterisks) and charged interactions with invariant Lys-378 (red arrowhead). The π-π interaction of ATP with Phe-633 is shown as an orange line in WT and AS PKCδ.