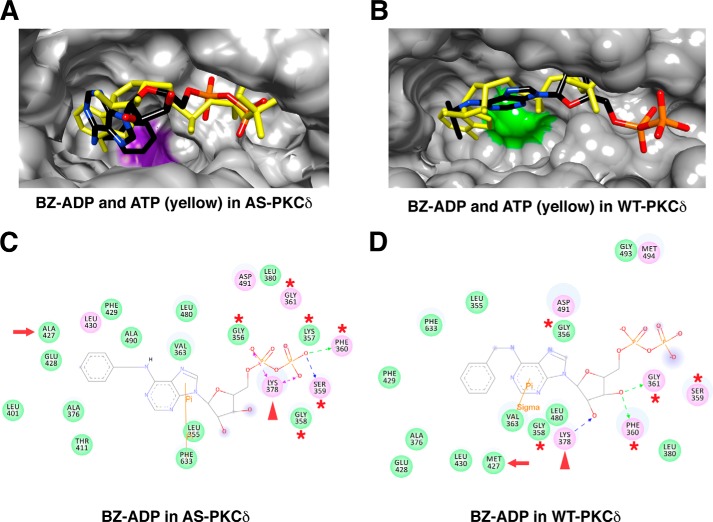
FIGURE 8.
Comparison of N6-(benzyl)-ADP (BZ-ADP) interactions in the nucleotide-binding pocket of AS and WT PKCδ. Shown are the superimposed structures of BZ-ADP (colored by atom type) and ATP (yellow) in AS (A) and WT PKCδ (B). Ala-427 is highlighted in purple in AS PKCδ, and Met-427 is highlighted in green in WT PKCδ. Shown are the BZ-ADP-interacting residues in the nucleotide-binding pocket of AS (C) and WT PKCδ (D). ATP interacts with the residues in the glycine-rich loop (red asterisks) and the invariant Lys-378 (red arrowheads). The gatekeeper is indicated by a red arrow.