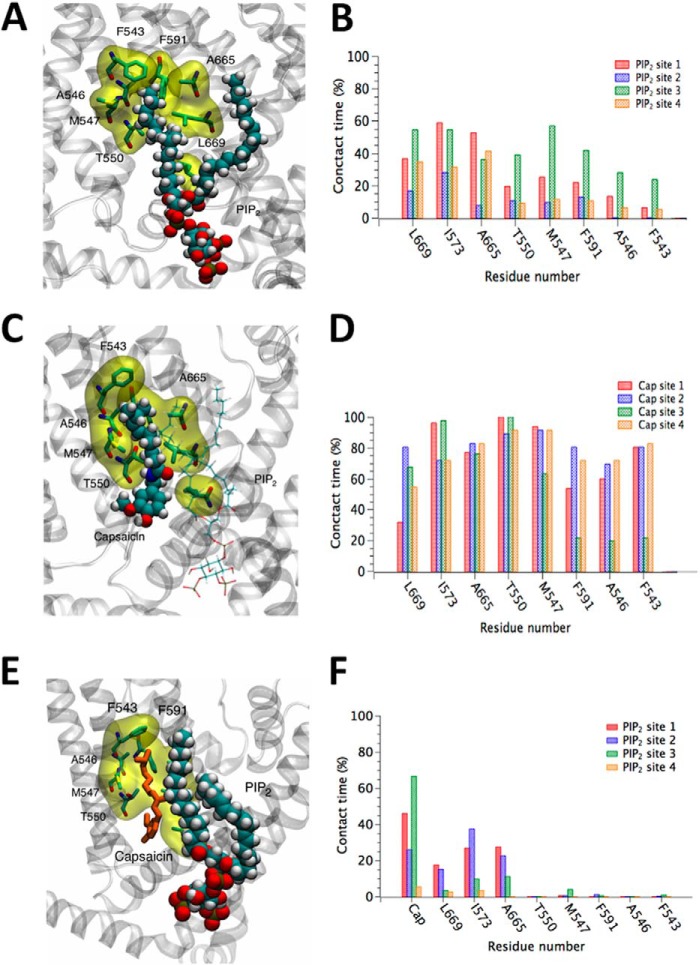
FIGURE 8.
Bound PI(4,5)P2 can partially occlude the nearby CAP binding site. A, snapshot from an MD simulation in which PI(4,5)P2 is bound to the binding site determined in the present work. Here, in the absence of CAP, an aliphatic chain of PI(4,5)P2 occupies the hypothesized CAP binding site. Atoms of PI(4,5)P2 are shown as spheres, while residues of TRPV1 that may be in contact with both bound PI(4,5)P2 and bound CAP are shown by a stick model and highlighted by a yellow surface. B, percentage of time in which atoms of PI(4,5)P2 make contact with specific residues of the CAP binding site in the absence of CAP. Occupancy of the CAP binding site by the aliphatic chain of PI(4,5)P2 is only partial, and it frequently exits and reenters this site. This suggests that bound PI(4,5)P2 may partially, but not completely, block the access of CAP to its binding site. C, snapshot from an MD simulation where both CAP and PI(4,5)P2 occupy their respective binding sites. Atoms of CAP are shown as spheres, while PI(4,5)P2 is represented by thin lines. D, percentage of time in which atoms of CAP make contact with specific residues of its binding site while PI(4,5)P2 is bound nearby. CAP stably occupies the hypothesized binding site. E, another snapshot from the same simulation represented in panel C, highlighting contact between PI(4,5)P2 and CAP. Atoms of PI(4,5)P2 are shown as spheres, while CAP is represented by thin orange tubes. F, percentage of time in which atoms of PI(4,5)P2 make contact with specific residues of the CAP binding site while occupied by CAP. The presence of CAP substantially reduces the occupation of the site by the PI(4,5)P2 aliphatic chain, which can be clearly seen by comparing to panel B.