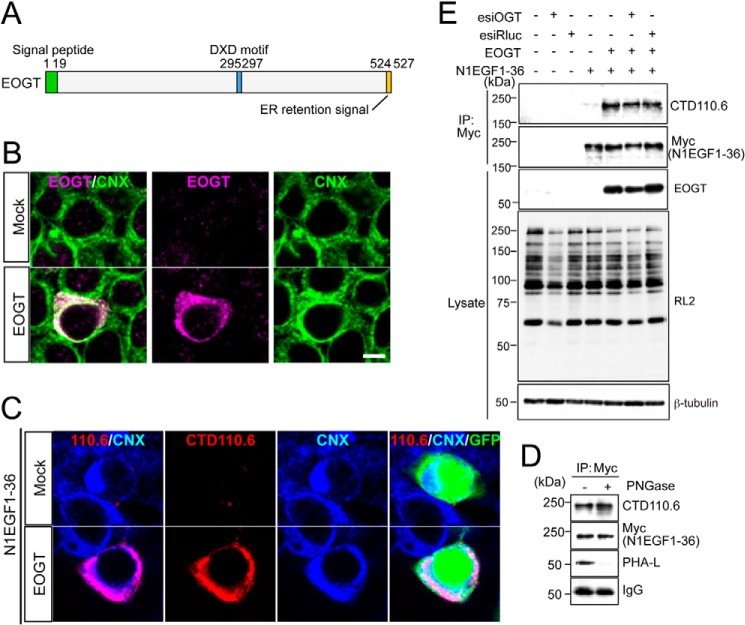
FIGURE 1.
O-GlcNAcylation occurs in the ER. A, schematic representation of the structure of EOGT. EOGT encodes a luminal ER protein that contains an amino-terminal signal peptide (green) and a carboxyl-terminal KDEL-like ER-retrieval signal (orange). A putative DXD motif involved in binding to the nucleotide sugar is shown in blue. B, immunostaining with anti-EOGT and calnexin (CNX) antibodies in EOGT- or mock-transfected HEK293T cells. C, N1EGF1–36-MycHis was expressed in HEK293T cells to monitor O-GlcNAcylation by EOGT. Cotransfectants of N1EGF1–36-MycHis and EOGT showed strong immunoreactivity to CTD110.6 antibody. In contrast, weak immunostaining was observed in cells expressing N1EGF1–36-MycHis alone. GFP expression indicates N1EGF1–36-MycHis-transfected cells. D, N1EGF1–36-MycHis was immunoprecipitated from the cell lysate of HEK293T cells expressing EOGT, untreated or deglycosylated with PNGase F, and immunoblotted with CTD110.6, anti-Myc tag antibody, or anti-mouse IgG antibody. Where indicated, lectin blotting with phytohemagglutinin-L (PHA-L) was performed. Note that PHA-L detects N-glycans on anti-Myc tag antibodies. E, N1EGF1–36-MycHis immunoprecipitated (IP) from the cell lysates of HEK293T cells co-transfected with or without EOGT together with esiRNA against OGT (esiOGT) or Renilla luciferase (esiRluc) was analyzed by immunoblotting (IB) with the indicated antibodies.