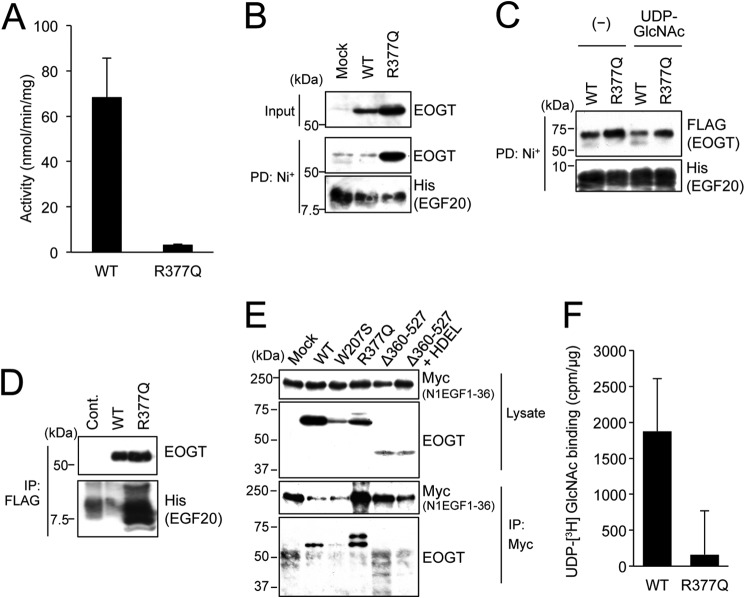
FIGURE 8.
The R377Q mutant exhibits lack of enzymatic activity and impaired UDP-GlcNAc binding. A, in vitro O-GlcNAc transferase assay was used to measure the enzymatic activity of the R377Q mutant. B, recombinant Notch EGF20:His was incubated with cell lysates from HEK293T mock transfectants or transfectants expressing EOGTWT or EOGTR377Q, affinity purified from the mixtures using Ni+ beads, and subjected to immunoblotting with indicated antibodies. C, purified EOGTWT or EOGTR377Q was incubated with recombinant Notch EGF20:His with or without UDP-GlcNAc, affinity purified, and subjected to immunoblotting with the indicated antibodies. D, Notch EGF20:His was incubated with cell lysates from CHO cells (Cont) or stable transfectants expressing FLAG-EOGTWT or FLAG-EOGTR377Q, immunoprecipitated (IP) with anti-FLAG antibody-conjugated beads, and subjected to immunoblotting as indicated. E, HEK293T cells were cotransfected to express N1EGF1–36-MycHis and the indicated EOGT constructs. Cell lysates were immunoprecipitated with anti-Myc tag antibody, and N1EGF1–36-MycHis·EOGT complexes were analyzed by immunoblotting with the indicated antibodies. F, lysates from HEK293T cells expressing FLAG-EOGTWT or FLAG-EOGTR377Q were incubated with UDP-[3H]GlcNAc and EGF20 on ice and immunoprecipitated using anti-FLAG antibody, after which the level of radioactivity was measured. Lysates from mock transfectants were similarly processed for use as a control. Vertical bars represent the range of values obtained from duplicate samples.