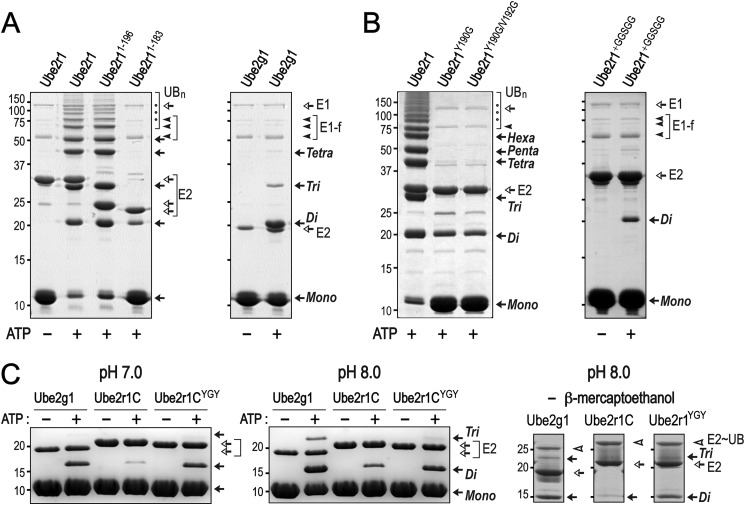
FIGURE 2.
E3-independent Lys-48 ubiquitylation activities of Ube2g1, Ube2r1, and Ube2r1 mutants. The in vitro ubiquitylation reactions were performed using E1 (∼0.5 μm), E2 (20 μm), and ubiquitin (0.2 mm) in a buffer containing 50 mm Tris-HCl (pH 7.5). Ubiquitin and the polyubiquitin products are marked Mono, Di, Tri, etc. A, Ube2r1 requires C-terminal residues 184–196 for wild-type activity. The activity of Ube2r11–183 (Ube2r1C) was much lower than that of either Ube2r1 or Ube2g1. B, the Ube2r1 mutant proteins (Y190G and Y190G/V192G) showed significant impairment in their in vitro ubiquitylation activities. The insertion of a GGSGG sequence between Val-183 and Pro-184 of Ube2r1 resulted in a complete loss of Lys-48 ubiquitylation activity. C, Ube2r1CYGY had Gln-105–Ser-106–Gly-107 in the acidic loop replaced by the Tyr-102–Gly-103–Tyr-104 sequence from Ube2g1. Although the Lys-48-ubiquitylation activity of Ube2r1C was lower than that of Ube2g1, Ube2r1CYGY showed activity to a level close to that of Ube2g1 (left panel). The formation of E2∼UB thioester adducts by Ube2g1, Ube2r1C, and Ube2r1CYGY was assessed by non-reducing SDS-PAGE analysis. Samples were prepared in SDS-PAGE loading buffer without β-mercaptoethanol 15 min after the ubiquitylation reaction was initiated with ATP. The three enzymes showed similar amounts of E2∼UB thioester intermediate formation (right panel).