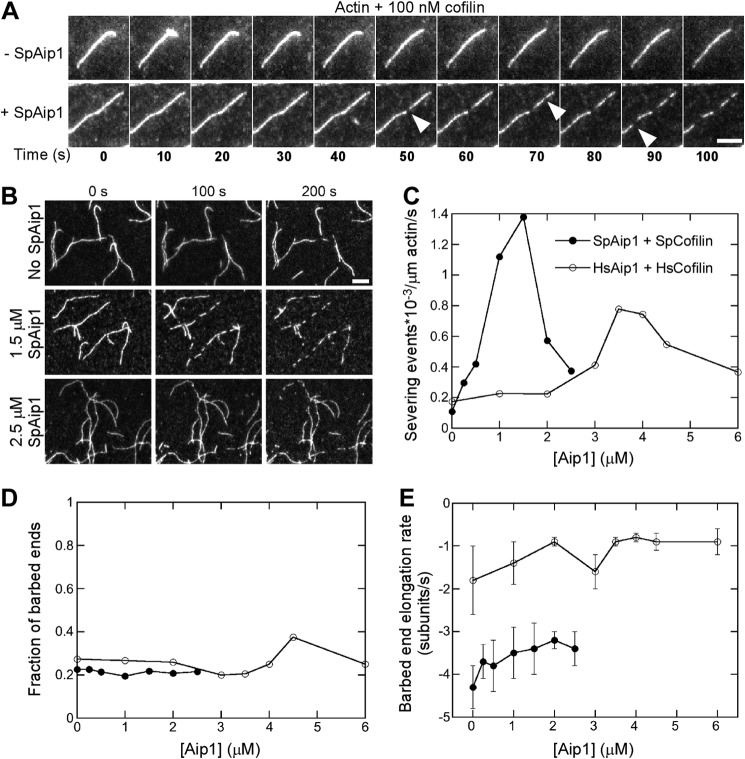
FIGURE 3.
Aip1 enhances actin filament severing by cofilin observed by TIRF microscopy in standard microscopy buffer. A and B, time series of images showing actin filament severing by 100 nm fission yeast cofilin with or without SpAip1. Bars, 5 μm. A, time lapse images at 10-s intervals of single actin filaments with 0 or 1.5 μm Aip1. B, images at 100-s intervals of a field of actin filaments being severed with 0, 1.5, or 2.5 μm SpAip1. Filament seeds were grown from 0.5 μm Mg-ATP-actin monomers in the presence of SpAip1, followed by a wash and replacement with 100 nm cofilin with SpAip1 in polymerization buffer without actin monomers. C, dependence of the severing activity of 100 nm fission yeast cofilin or 7 μm human cofilin 1 on the concentration of SpAip1 (filled circles) or HsAip1 (open circles). D, dependence of the fraction of new barbed ends that do not shorten following severing on the concentration of SpAip1 (filled circles) and HsAip1 (open circles). E, dependence of the depolymerization rates of new barbed ends created by fission yeast cofilin severing events on the concentration of SpAip1 (filled circles) and HsAip1 (open circles). The data were collected as for A. The error bars are ±1 standard deviation of the mean elongation rates of at least 6–10 filaments for experiments done with SpAip1 or HsAip1. The depolymerization rates do not vary significantly across this range of concentrations of either SpAip1 or HsAip1 (p > 0.1, single factor analysis of variance test).