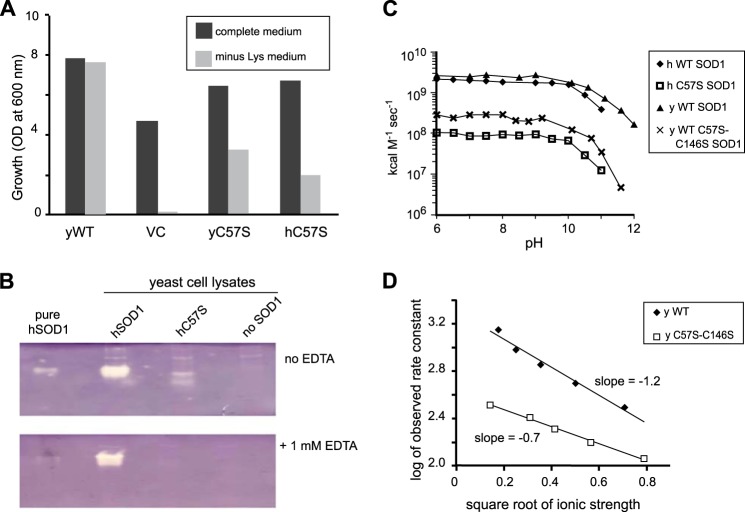
FIGURE 8.
Disulfide mutant yeast and human SOD1s are active in vitro and in vivo. A, yeast growth trials. The indicated yeast or human C57S sod1 genes, on overexpression plasmid pRS424 or vector control (VC), were transformed into EG118 sod1Δ yeast. Cultures were inoculated, in complete media or minus-lysine media, to A600 = 0.05 and grown for 24 h at 30 °C with 220 rpm shaking. The results indicate that both yeast and human disulfide mutant SOD1 partially rescue yeast from the sod1Δ lysine auxotrophy. B, activity by native activity gel. The presence of white bands indicates both human wild type and disulfide mutant (C57S) SOD1 are enzymatically active in cell lysates. C, activity by pulse radiolysis. Rate constants are shown for dismutation of superoxide as a function of pH for human and yeast wild type and disulfide mutant SOD1. Rates were normalized for the copper content of each enzyme. D, ionic strength trials. Using pulse radiolysis, results indicate that disulfide mutant SOD1 is less sensitive (lower slope) to increased ionic strength than is wild type SOD1.