FIGURE 5.
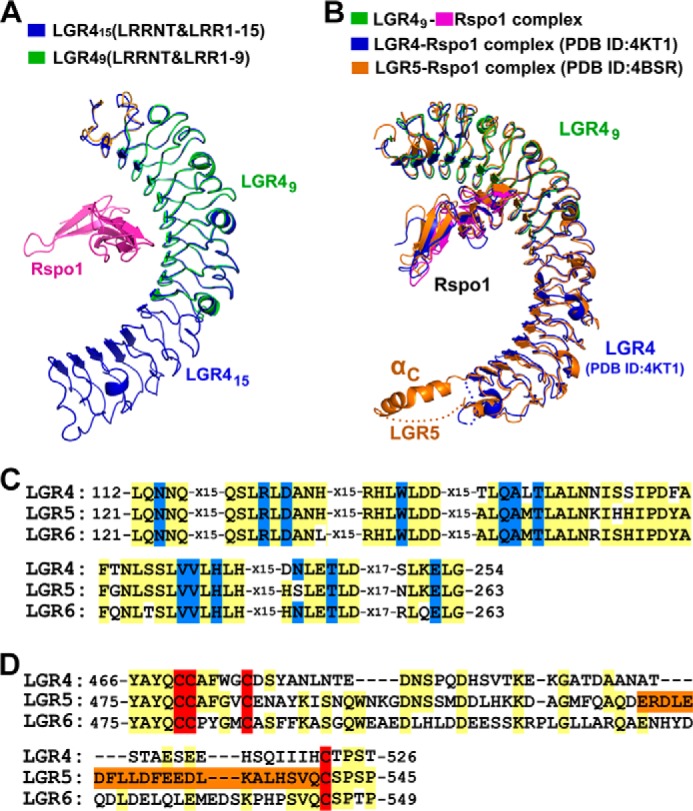
Comparing LGR4 with LGR5 and LGR6. A, structural comparison of Rspo1-bound LGR49 and ligand-free LGR415. LGR49 (LRRNT and LRR(1–9)) was superimposed with LGR415 (LRRNT and LRR(1–15)). The VLR modules in LGR415 and LGR49 were omitted in structural superimposition. LGR49 and Rspo1 are colored as in Fig. 2B. LGR415 is colored in blue. B, structural comparison of the LGR49-Rspo1 complex with the previously determined LGR4-Rspo1 and LGR5-Rspo1 structures. The LGR49-Rspo1 complex is colored in green (LGR49) and purple (Rspo1). The previously determined LGR4-Rspo1 and LGR5-Rspo1 structures are colored in blue and orange, respectively. C, sequence alignment of the Rspo1-binding residues. The Rspo1-binding residues are colored in blue. All the Rspo1-binding residues of LGR4 are conserved in LGR5 and LGR6. D, sequence alignment of the residues around αC in the LRRCT regions. The cysteine residues of disulfide bonds in the LRRCT regions are colored in red. The sequence of the αC helix of LGR5 is colored in orange.
