FIGURE 3.
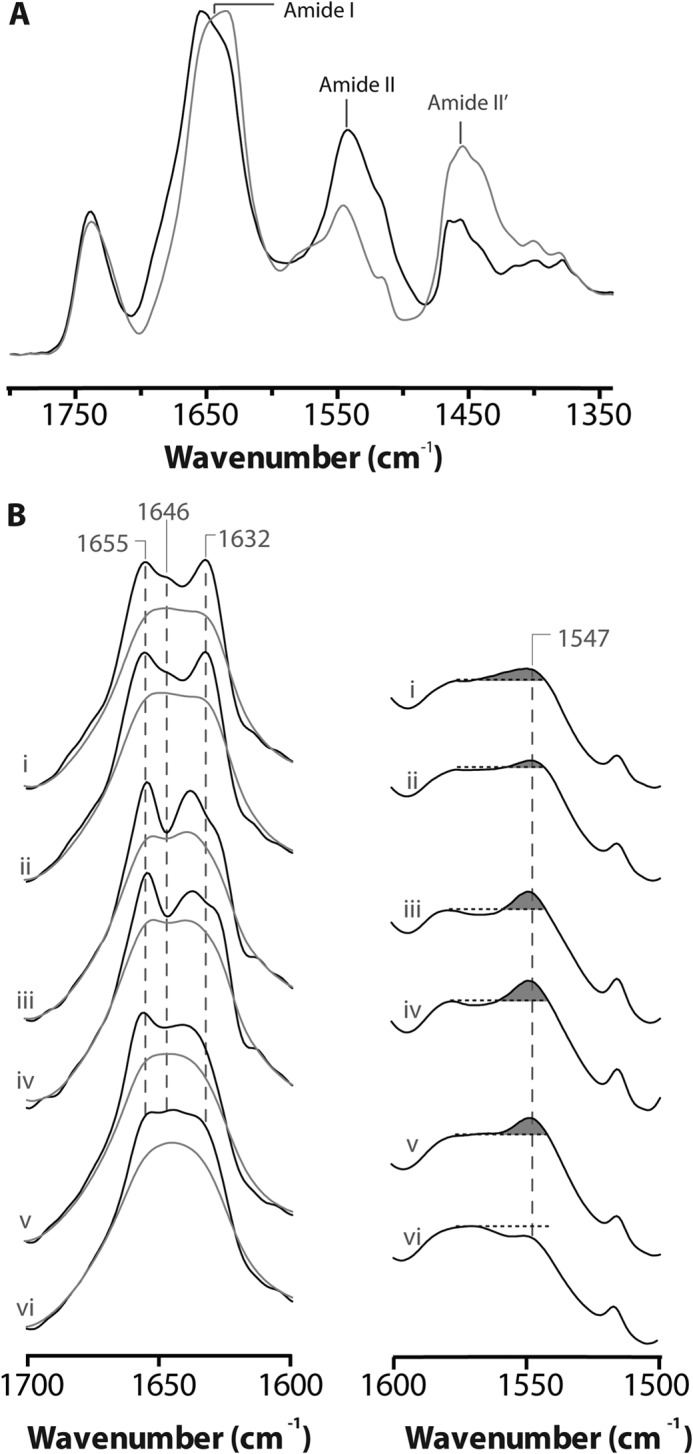
Structural comparisons of membrane reconstituted ELIC, GLIC and the nAChR as probed by infrared spectroscopy. A, infrared spectra of aso-ELIC recorded after gentle drying from 1H2O buffer (black) and immediately after the addition of 2H2O (gray). Note the immediate changes in amide I band shape (from 1700 to 1600 cm−1) and the immediate decrease in amide II band intensity (1547 cm−1), both indicative of the rapid peptide N-1H/N-2H exchange of solvent-exposed peptide hydrogens. B, the structure-sensitive amide I (left) and amide II (right) bands in infrared spectra recorded after 24 h of equilibration in 2H2O at 4 °C from aso-ELIC (i) and PC-ELIC (ii) and after 72 h equilibration in 2H2O at 4 °C for aso-GLIC (iii), PC-GLIC (iv), aso-nAChR (v), and PC-nAChR (vi) (11, 31). The amide I bands are shown both before (gray) and after resolution enhancement (black). Spectra are the averages of at least three spectra recorded from two different purifications/reconstitutions. See Fig. 2 for details.
