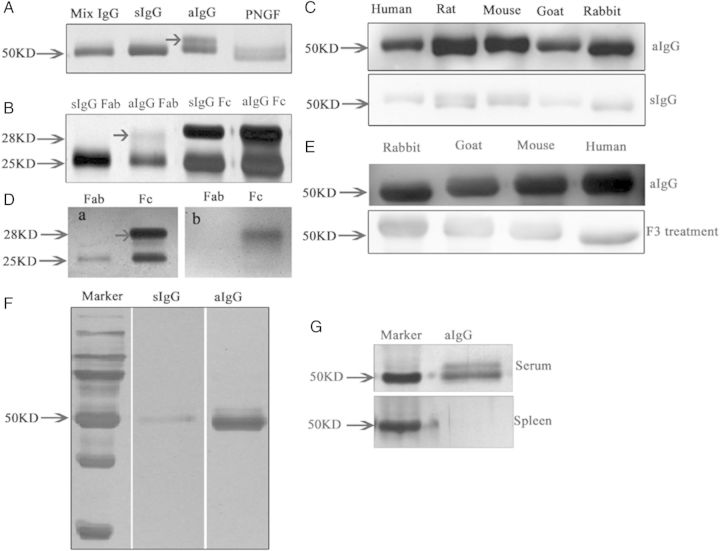
Figure 5.
Electrophoresis of asymmetrically glycosylated IgG (aIgG) and symmetrically glycosylated IgG (sIgG) extracted from placenta. (A) Silver stain of IgG extracted from the human placenta showing aIgG had a double band (arrow) at around 50 kDa. When the aIgG was treated with deglycosylation enzyme PNGF, the double band became a single band. (B) Sliver stain showing that the aberrant glycan is located in one of the Fab arms. (C) Western blot of aIgG and sIgG showing that aIgG reacted to IgGs from human, rat, mouse, goat and rabbit, while sIgG only reacted very weakly to IgG molecules of different species. (D) a, silver stain of Fab and Fc (arrow) fragment; b, western blot of aIgG showing that aIgG reacted to the Fc fragment of the other IgGs but not to the Fab fragment. (E) When the aIgG was digested with the enzyme to remove the glycan, the reactivity to IgG molecules was significantly weakened. (F) In a separate experiment where the aIgG and sIgG were coated onto magnetic beads and reacted to human IgG, the aIgG trapped significantly more IgG than the sIgG indicating that aIgG was indeed capable of reacting to other IgG molecules. (G) Silver stain showing that aIgG can be detected in the serum but not in the spleen of normal adult rat.