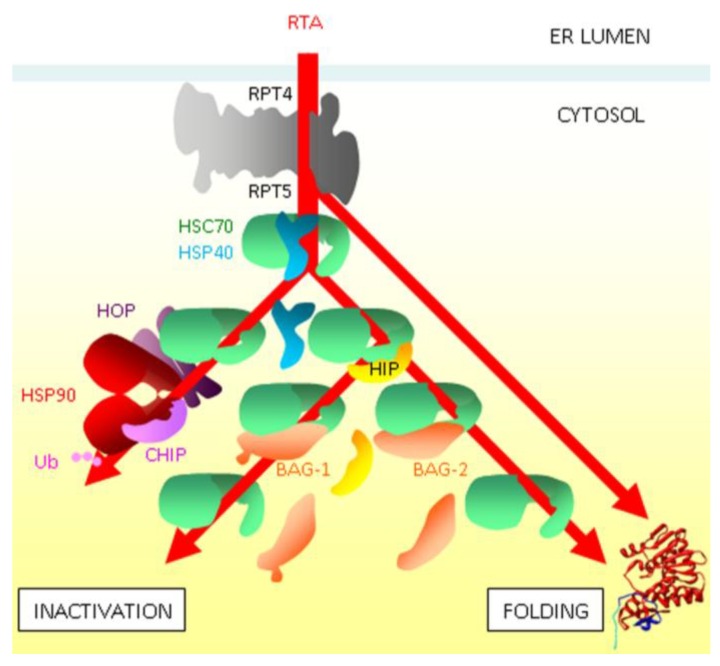
Figure 7.
Post-dislocation scrutiny by a network of chaperones determines the cytosolic fate of RTA [38,40,81]. Following extraction from the ER by the RPT4 subunit of the proteasome, interactions with RPT5 allow RTA to recover activity. The role of Hsc70/Hsp40 interactions may be continuous scrutiny of RTA, preventing aggregation, and inactivation and activation (folding) fates follow from release of RTA from this complex. Transfer to Hsp90 via the Hsc70-Hsp90 operating protein HOP leads to CHIP-mediated ubiquitylation (Ub, ubiquitin) and inactivation of RTA. The Hsc70-interacting protein HIP stabilizes the Hsc70:RTA complex, and subsequent release by the BAG family guanine nucleotide exchange factors BAG-1 and BAG-2 leads to inactivation or folding respectively.