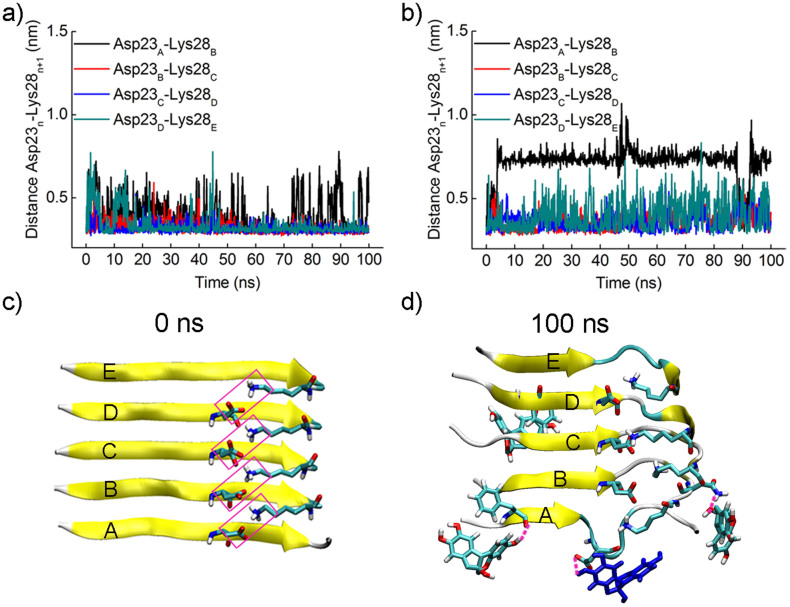
Figure 4. Brazilin disrupts the salt bridge Asp23-Lys28.
The distances between the mass center of the carboxyl group of Asp23 and the amino group of Lys28 in two adjacent chains were monitored as a function of simulation time (a) in the absence and (b) presence of brazilin. (c) The salt bridges between each chain and its adjacent chain within the initial Aβ17–42 pentamer. The salt bridges are framed in pink. (d) The snapshot of the salt bridge between chains A and B disrupted by brazilin molecules at 100 ns. The three hydrogen bonds observed between brazilin and Aβ17–42 pentamer are represented using pink dash lines. The brazilin molecules interacting with Asp23 via hydrogen bonding are colored in blue. The main chain of Aβ17–42 is shown by a yellow NewCartoon model. Atoms of brazilin and the side chains of some residues are colored red for oxygen, white for hydrogen, and green for carbon. The snapshots are plotted by visual molecular dynamics (VMD) software (http://www.ks.uiuc.edu/Research/vmd/).