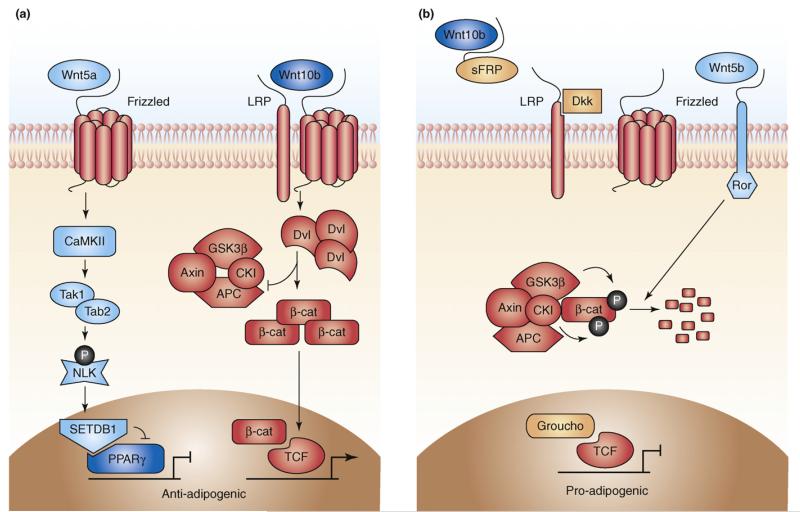
Figure 3.
Adipogenesis and WNT signalling. (a) In mesenchymal precursor cells, Wnt10b (and Wnt10a) binding to frizzled (FZD1) receptors and LRP5/6 co-receptors leads to dishevelled (DVL) phosphorylation and Axin degradation. This, in turn, results in hypophosphorylation of β-catenin, which accumulates in the cytoplasm and translocates to the nucleus, where it binds to TCF/LEF transcription factors to activate WNT target genes and inhibit adipogenesis by suppressing CEBPA and PPARG. Non-canonical WNT signalling, perhaps through Wnt5a acting via FZD2, might also inhibit adipocyte differentiation. Wnt5a stimulates a mitogen-activated protein kinase cascade mediated through CAMKII-TAK1-TAB2 (TAK1, TGFβ-activated protein kinase-1; TAB2, TAK1-binding protein-2) to activate Nemo-like kinase (NLK). NLK, in turn, phosphorylates a histone methyltransferase, SET domain bifurcated-1 (SETB1), leading to the assembly of a co-repressor complex, which inactivates ligand-bound PPARG through histone methylation. (b) Adipocyte differentiation is associated with secretion of sFRPs and Dkk1, which inhibit Wnt10b (and Wnt10a) signalling. sFRPs directly bind and sequester Wnt10b, whereas Dkk1 inhibits WNT signalling by binding as a high-affinity antagonist to LRP co-receptors. In the absence of WNT ligands, cytoplasmic β-catenin is recruited to a destruction complex that contains Axin and APC, which facilitates the GSK3β-dependent-phosphorylation and proteasomal degradation of β-catenin. In contrast to Wnt10b, Wnt5b promotes adipogenesis by inhibiting WNT/β-catenin signalling, perhaps by acting through ROR receptors.