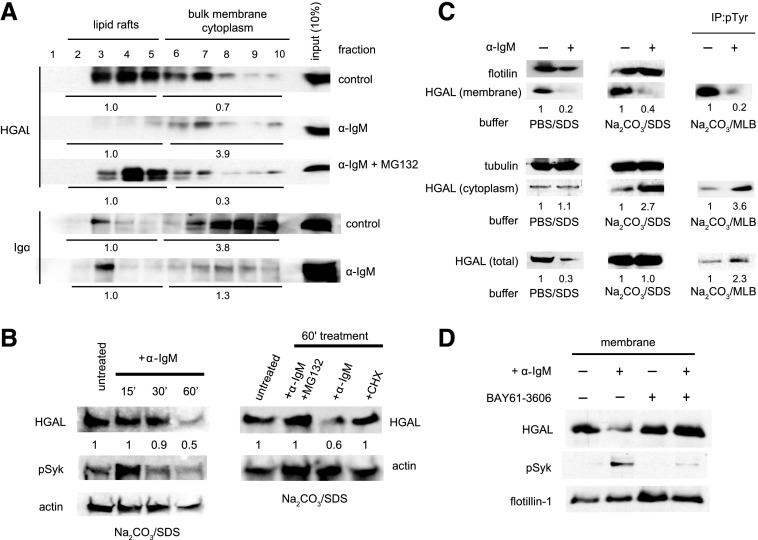
Figure 5.
BCR stimulation induces HGAL translocation from lipid rafts to cytoplasm and leads to HGAL degradation. (A) Raji cells were left unstimulated (“control”) or stimulated for 5 minutes in the presence or absence of MG132 (30 μM) with anti-human IgM F(ab)2 (a-IgM). Cellular lysates were subjected to detergent-free fractionation, and equal volumes of each fraction were immunoblotted with the indicated antibodies. Total densitometry in lipid raft fractions (2-5) and in bulk membrane/cytoplasm fractions (6-10) was measured, and relative distribution of HGAL and Igα was compared by randomly assigning value 1 to total densitometry measured in the lipid raft fractions (2-5). Results are representative of 3 independent experiments. (B) Left image: Raji cells were left unstimulated (“untreated”) or were stimulated with a-IgM for 15, 30, or 60 minutes. Cellular lysates were immunoblotted with HGAL, pSyk (Y525/526), or actin. Right image: Raji cells were left untreated or treated for 1hour with a-IgM with or without proteasome inhibitor MG132 (30 μM) or with cycloheximide (CHX; 40 μM) alone. Densitometry was measured for HGAL and normalized for actin content. The value 1 was assigned to the untreated sample. (C) Raji cells were left unstimulated (−) or stimulated (+) with a-IgM for 5 minutes. Membrane and cytoplasm fractionation in either PBS (pH 7.4) or sodium carbonate (Na2CO3) buffer (pH 11) was performed as described in the supplemental Materials and Methods. The fractions were immunoblotted with the indicated antibodies. HGAL (total) represents HGAL contents of whole-cell lysate before separation into cytoplasmic and membrane fractions. Membrane and cytoplasm fractions of Raji cells in sodium carbonate buffer were diluted in mild lysis buffer (MLB) and used for immunoprecipitation (IP) with monoclonal antibody to phosphotyrosine (pTyr; 4G10), followed by immunoblotting with antibody to HGAL. Densitometry before and after IgM treatment was measured and compared by assigning value 1 to the nontreated sample. Results are representative of 3 independent experiments. (D) Raji cells untreated (−) or preincubated (+) with BAY61-3606 (20 nM) for 30 minutes at 37°C were left unstimulated (−) or stimulated (+) with a-IgM for 5 minutes. Membrane and cytoplasm fractionation in sodium carbonate buffer (pH 11) was performed as in panel C, followed by immunoblotting with antibodies to HGAL, pSyk (Y525/526), and flotillin-1. Only membrane fractions are represented. SDS, sodium dodecyl sulfate.