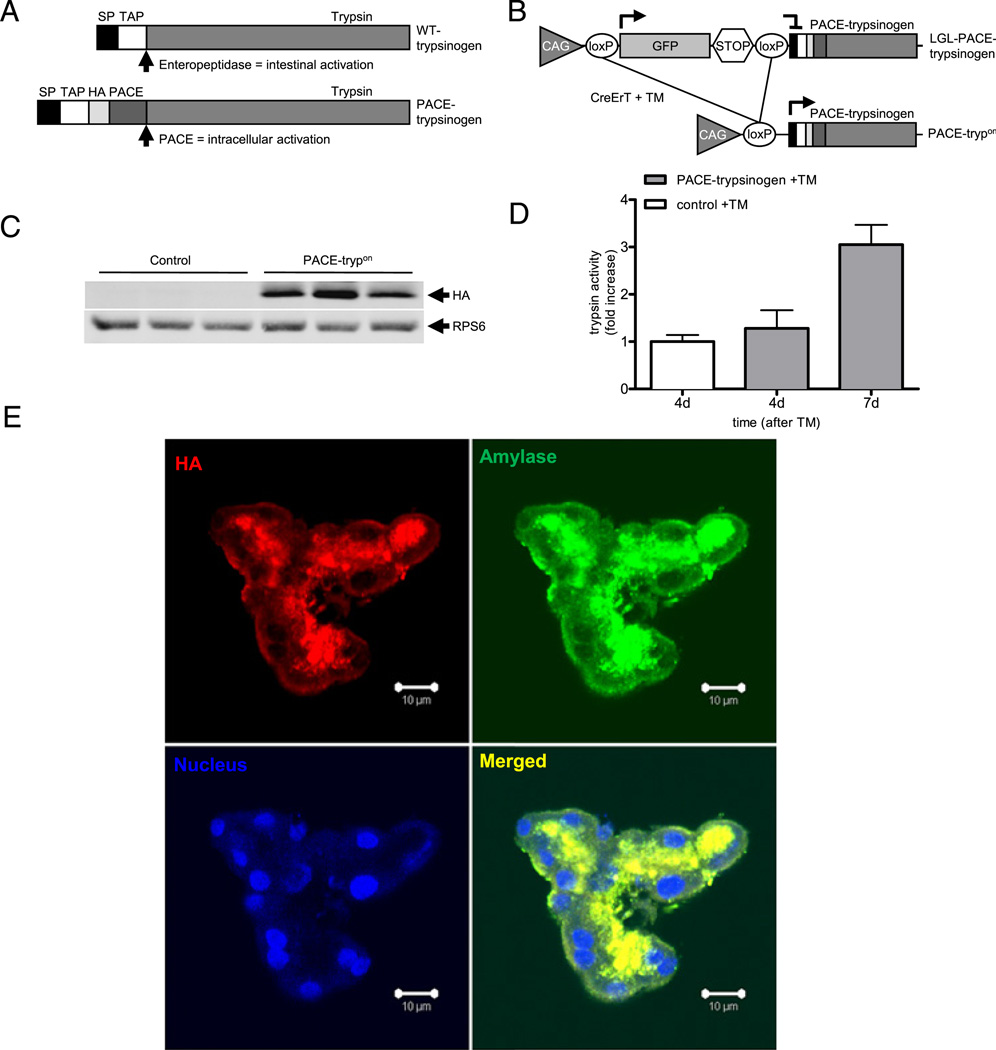
Figure 1.
Principle of PACE (paired basic amino acid-cleaving enzyme)-mediated trypsinogen activation in transgenic mice. (A) Wild-type (WT) trypsinogen is activated by enteropeptidase in the duodenum. Insertion of a PACE recognition site allows the genetically modified trypsinogen (PACE–trypsinogen) to be cleaved and activated by PACE within acinar cells. (SP, signal peptide; TAP, trypsinogen activation peptide; HA, haemagglutinin tag). (B) Induction of Cre activity in LGL-PACE-trypsinogen×CreErT double transgenic mice (PACE-trypon) removes the GFP-stop cassette. (CAG, chimeric human CMV-IE enhancer and chicken β-actin promoter; GFP, green fluorescent protein). (C) PACE–trypsinogen was expressed in PACE-trypon mice as shown in this western blot (1 week after induction). (D) Trypsin activity levels in pancreatic homogenates of PACE-trypon mice were significantly elevated 1 week after induction with tamoxifen (TM) (n=4, *p<0.05). (E) PACE–trypsinogen with an HA tag at its C-terminus was expressed in pancreatic acinar cells. The HA tag (red) and amylase (green) were visualised by immunofluorescence using anti-HA and antiamylase antibodies. Co-localization (yellow) of these two proteins indicated that active trypsin was sorted to the secretory pathway (scale bar 10 µm).