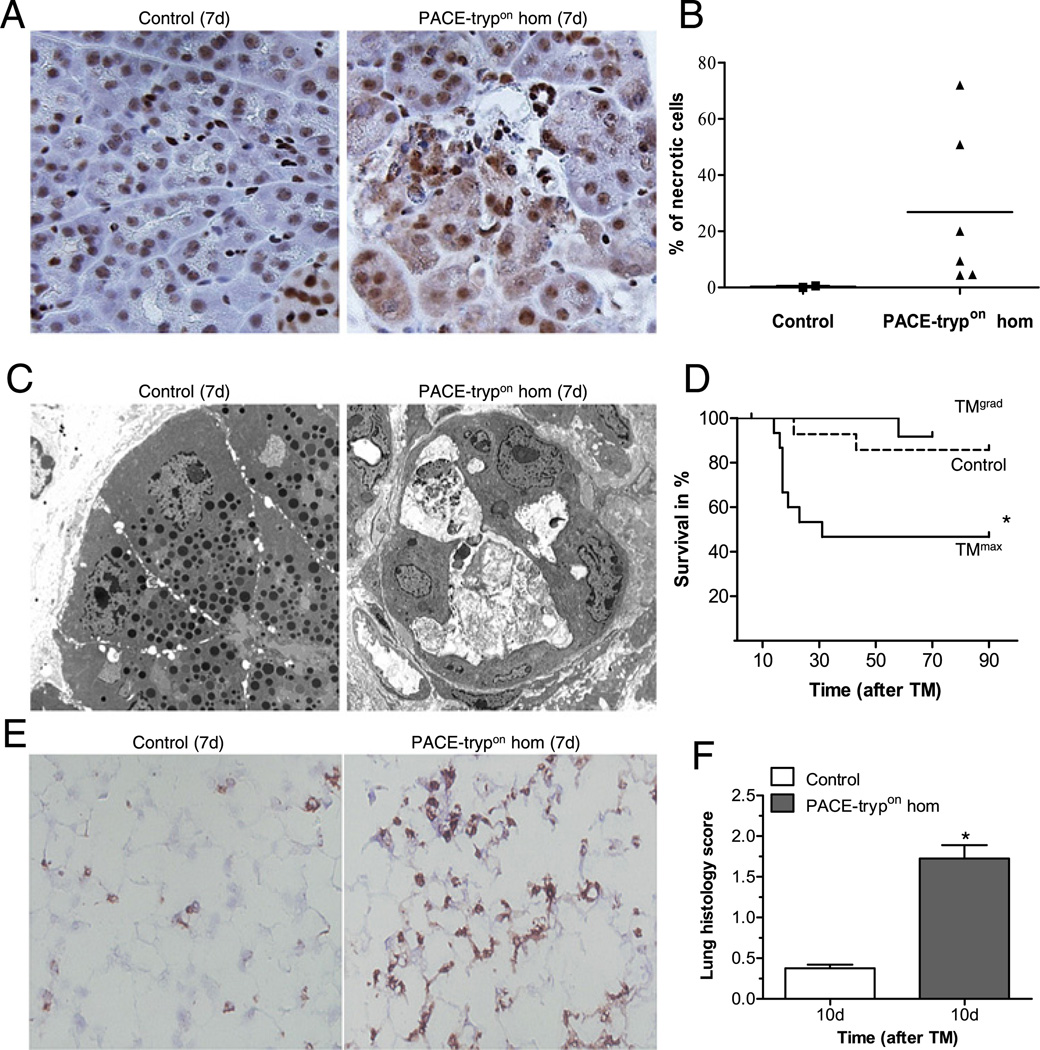
Figure 5.
PACE-trypon mice displayed acinar cell necrosis and increased mortality. (A) Seven days after tamoxifen (TM) treatment, formalin-fixed pancreata were sectioned and stained with anti-high mobility group protein B1 (HMGB1) antibody. Prominent cytosolic translocation of HMGB1, a marker of necrosis, was frequently found in homozygous PACE-trypon mice but not controls (400×). (B) Counting cells with HMGB1 cytosolic translocation allowed quantitative analysis of necrotic cells in the pancreas of TM-treated control and homozygous PACE-trypon mice (7d). (C) Electron microscopy (4000×) demonstrated that control animals had no signs of acinar cell damage with normal endoplasmic reticulum and intact zymogen granules (left panel). In contrast, homozygous PACE-trypon mice (7d) showed abundant intercellular debris and severely damaged acinar cells leaking their content into the enlarged lumen (right panel). (D) Mortality in PACE-trypon mice with maximal-rapid induction with TM (TMmax) was significantly higher than those treated with gradual-repetitive induction with TM (TMgrad; control, n=15; TMgrad, n=12; TMmax, n=15; p<0.05). (E) Prominent neutrophil infiltration in the lung of homozygous PACE-trypon mice (7d) was identified by Gr-1 staining (200×). (F) Examination of lung tissue at a time point where mortality was expected to occur (10 days) revealed significantly elevated histomorphological scores (n=4, p<0.01).