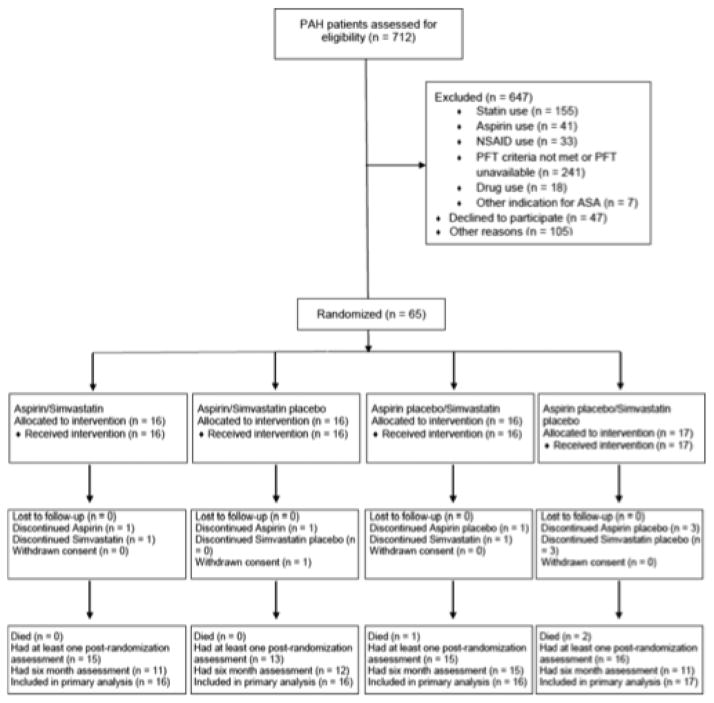
Figure 2. Factorial study design used in the ASA-STAT trial involving patients with pulmonary arterial hypertension (PAH).
Patients were randomly assigned in a 1:1:1:1 ratio by a Web-based computerized system to: (1) aspirin 81 mg once daily plus simvastatin 40 mg once daily, (2) aspirin 81 mg once daily plus placebo simvastatin once daily, (3) placebo aspirin placebo once daily plus simvastatin 40 mg once daily, or (4) placebo aspirin once daily plus placebo simvastatin once daily. NSAID, nonsteroidal anti-inflammatory drug; PFT, pulmonary function test; and ASA, aspirin. Reproduced with permission from51.