Figure 3. Schematic representation of a crossover study design.
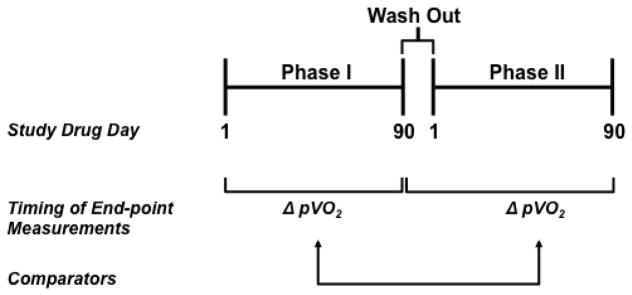
In Phase I, patients are randomized to treatment with placebo or study drug and testing relevant to the study end-points, such as peak volume of oxygen consumption (pVO2), occurs at study drug day 1 (i.e., baseline) and day 90. Following a 21-day drug wash out period, subjects enter Phase II of the trial, which is characterized by cross-over to therapy opposite of Phase I. Repeat end-point assessment will be performed at study drug day 90 of Phase II. Change in performance on end-points from study drug day 1 at study drug day 90 (Phase I) are compared to change in performance from study drug day 1 at study drug day 90 (Phase II), using a 2-sided, paired Student’s t test.
