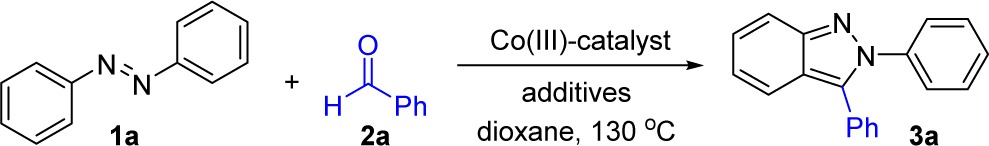
Table 1. Optimization of Reaction Conditions for N-Aryl-2H-indazole Synthesis with [Cp*CoCl2]2a.
| entry | Co(III) source (mol %) | Ag salt (mol %) | acetate additive (mol %) | yield (%)b |
|---|---|---|---|---|
| 1 | 4 (10) | 2 | ||
| 2 | [Cp*CoCl2]2 (5) | AgPF6 (20) | 3 | |
| 3 | [Cp*CoCl2]2 (5) | AgSbF6 (20) | 1 | |
| 4 | [Cp*CoCl2]2 (5) | AgOAc (20) | ||
| 5 | [Cp*CoCl2]2 (5) | AgB(C6F5)4 (20) | 51 | |
| 6 | [Cp*CoCl2]2 (5) | AgB(C6F5)4 (25) | 61 | |
| 7 | [Cp*CoCl2]2 (5) | AgB(C6F5)4 (25) | AgOAc (20) | 97 (91)c |
| 8 | [Cp*CoCl2]2 (2.5) | AgB(C6F5)4 (12.5) | AgOAc (10) | 63 |
| 9 | [Cp*CoCl2]2 (1) | AgB(C6F5)4 (5) | AgOAc (4) | 44 |
| 10 | [Cp*CoCl2]2 (2.5) | AgB(C6F5)4 (12.5) | KOAc (10) | 54 |
| 11d | [Cp*CoCl2]2 (2.5) | AgB(C6F5)4 (12.5) | AgOAc (10) | 45 |
| 12 | AgB(C6F5)4 (25) | AgOAc (20) |
Conditions: 2a (1.0 equiv), 1a (2.0 equiv) in 1,4-dioxane (2.0 M) for 24 h.
Determined by GC analysis relative to tetradecane as an external standard.
Isolated yield at 0.20 mmol scale.
Reverse stoichiometry: 2a (2.0 equiv), 1a (1.0 equiv).
