Table 2. Optimization of Reaction Conditions for Indazole Synthesis with Catalyst 5a.
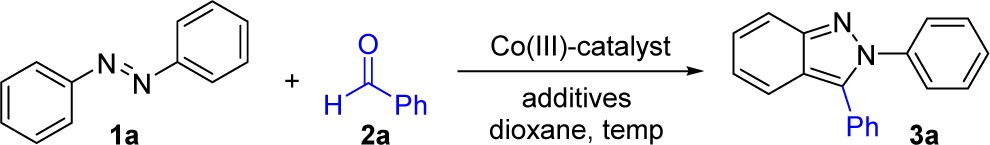
| entry | preformed Co(III) catalyst (mol %) | additive | temp (°C) | yield (%)b |
|---|---|---|---|---|
| 1 | 4 (10) | 130 | 2 | |
| 2 | 5 (10) | 130 | 31 | |
| 3 | 5 (10) | AgOAc (10 mol %) | 130 | 82 |
| 4 | 5 (10) | AgOAc (20 mol %) | 130 | 72 |
| 5 | 5 (10) | Cu(OAc)2 (10 mol %) | 130 | 71 |
| 6 | 5 (10) | Cu(OAc)2 (20 mol %) | 130 | 25 |
| 7 | 5 (10) | Cu(OAc)2(H2O)4 (10 mol %) | 130 | 75 |
| 8 | 5 (10) | Cu(OAc)2(H2O)4 (20 mol %) | 130 | 50 |
| 9 | 5 (10) | H2O (50 mol %) | 130 | 45 |
| 10 | 5 (10) | H2O (1.0 equiv) | 130 | 53 |
| 11 | 5 (10) | H2O (2.0 equiv) | 130 | 27 |
| 12 | 5 (10) | AcOH (10 mol %) | 130 | 85 |
| 13 | 5 (10) | AcOH (10 mol %) | 100 | 87 |
| 14c | 5 (10) | AcOH (10 mol %) | 100 | 90 (83)d |
| 15 | 5 (10) | AcOH (50 mol %) | 130 | 71 |
| 16 | 5 (10) | PivOH (10 mol %) | 130 | 80 |
| 17 | 5 (5) | AcOH (5 mol %) | 130 | 67 |
| 18 | 5 (5) | PivOH (5 mol %) | 130 | 68 |
Conditions:2a (1.0 equiv), 1a (2.0 equiv) in 1,4-dioxane (2.0 M) for 24 h.
Determined by GC analysis relative to tetradecane as an external standard.
Reaction performed on the benchtop under nitrogen.
Isolated yield at 0.20 mmol scale.
