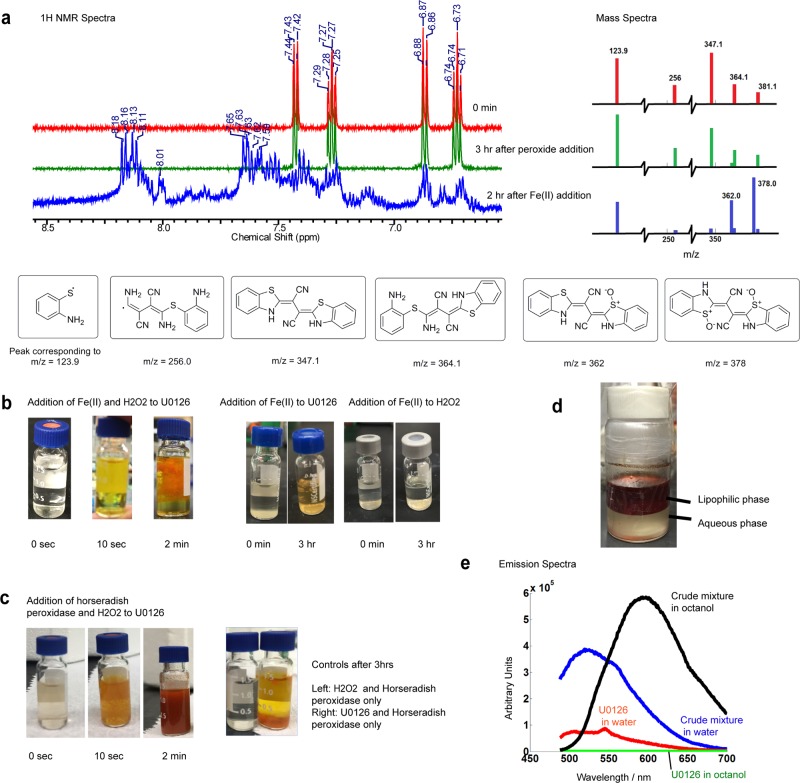
Figure 4.
U0126 directly reacts with hydroxyl radicals produced by Fenton’s reaction and horseradish peroxidase (HRP). (a) 1H NMR and mass spectrometry show that U0126 does not react with H2O2 directly. Addition of Fe(II) results in the appearance of downfield peaks in NMR and new peaks of m/z at 362 and 378 in mass spectra. (b) Mixing U0126, H2O2, and Fe(II) in vitro (Fenton reaction) shows bright orange precipitation within 2 min. Control experiments show that mixing U0126 with Fe(II) sulfate results in a faint yellowish color after 3 h, while mixing H2O2 and Fe(II) does not induce any color change. (c) Mixing U0126, H2O2, and horseradish peroxidase in vitro produces an orange-brown precipitate within seconds. (d) Lipophilicity assay of the reaction products shows that the intense orange-brown compound in the lipophilic phase (n-octanol) as compared to the aqueous phase in the bottom layer. (e) Fluorescence emission spectra of the crude reaction mixture in the n-octanol phase and in the water phase.