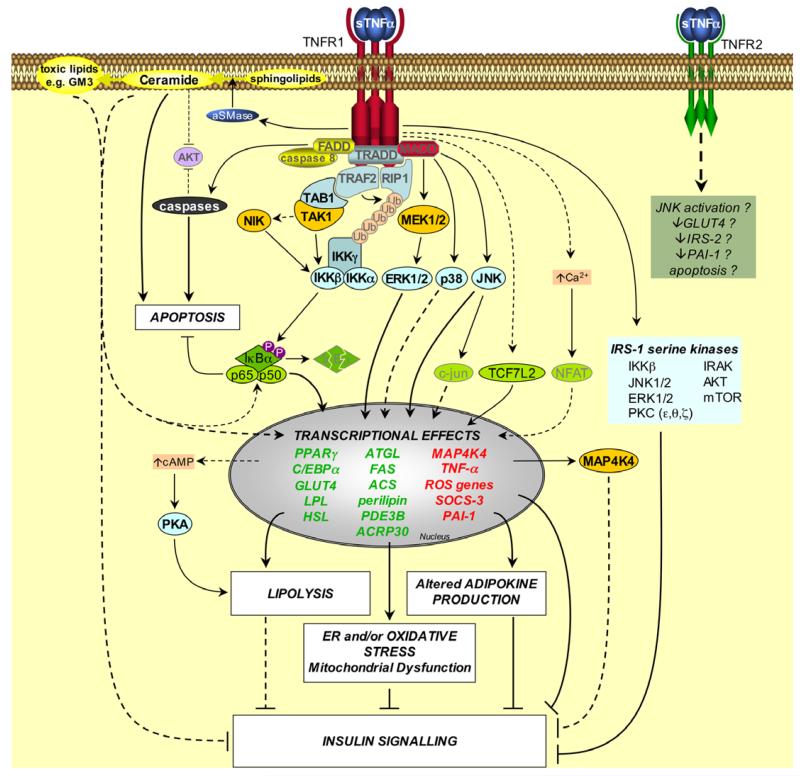
Fig. 1. Signalling pathways induced by TNF-α in adipose tissue.
Upon TNFR1 activation the adapter molecule TRADD (TNFR-associated death domain protein) interacts with the TNFR1-DD. TRADD recruits downstream adapter molecules such as Fas-associated death domain protein (FADD), TNFR-associated factor 2 (TRAF2), receptor-interacting protein 1 (RIP1) and mitogen-activated protein kinase (MAPK)-activating death domain protein (MADD). These adapters then mediate the activation of divergent downstream signalling pathways. Apoptosis: FADD recruits caspases such as caspase 8 to TNFR1, thereby forming the TNFR1 death-inducing signalling complex (DISC). This complex activates downstream caspases, leading to apoptosis. Ceramide production: the TNFR1-DD activates acidic sphingomyelinase (aSMase), which hydrolyses sphingolipids to produce ceramide. Ceramides can be converted into toxic lipids, such as the ganglioside GM3. Ceramides and gangliosides may then mediate transcriptional effects, possibly by activation of NFκB. Ceramides can also stimulate apoptosis and impair insulin signalling, and both ceramide and caspases may inhibit AKT. NFκB activation: TRAF2 and RIP1 mediate NFκB activation by recruiting the IKK (inhibitor of NFκB (IκB) kinase) complex to TNFR1. TRAF2 interacts with IKKβ and IKKα, and also promotes the K63-linked polyubiquitination of RIP1 (Ub, ubiquitin); Ubiquitinated RIP1 binds to IKKγ. TRAF2 also recruits TAK1 (TGFβ-activated kinase 1) to TNFR1 via an interaction with TAB1 (TAK1 binding protein 1), which may result in activation of TAK1. This also brings TAK1 into close proximity of the IKK complex. TAK1 may activate IKKβ either by phosphorylating it directly, or by activating NIK (NFκB-inducing kinase), which then phosphorylates IKKβ. Active IKKβ phosphorylates IκBα on conserved serines, leading to its polyubiquitination and proteasomal degradation. This liberates the bound NFκB dimers (e.g. the prototypical p65/p50 heterodimer), which translocate into the nucleus to mediate transcriptional effects. MAPK activation: TNF-α activates various MAPKs in adipocytes, including ERK1/2, p38 MAPK and JNK, possibly via the adapter MADD. ERK1/2 and JNK can each regulate transcription by suppressing the activity of PPARγ. JNK may also exert transcriptional effects via c-jun. JNK, ERK1/2, IKKβ and other kinases have also been implicated in TNF-α-induced serine phosphorylation of IRS-1, which suppresses insulin signalling. Other effects: TNF-α can activate PKA (protein kinase A) by increasing cAMP levels, possibly via transcriptional effects. TNF-α can activate the transcription factor NFAT via Ca2+, however this has not been established in adipocytes. In preadipocytes TNF-α can stimulate transcription by TCF7L2 (transcription factor 7-like 2). The transcriptional effects of TNF-α promote ER stress, oxidative stress and mitochondrial dysfunction, lipolysis and altered adipokine expression, thereby compromising insulin signalling and adipocyte lipid metabolism. TNFR2 may also mediate some effects of TNF-α in adipose tissue, albeit by unknown mechanisms. Factors that have not been directly established in TNF-α-induced signals in adipocytes are faded. Dashed lines indicate effects that are mediated by indirect or unestablished mechanisms. Question marks indicate effects that remain to be fully established. Genes written in red or green are upregulated or downregulated by TNF-α, respectively.