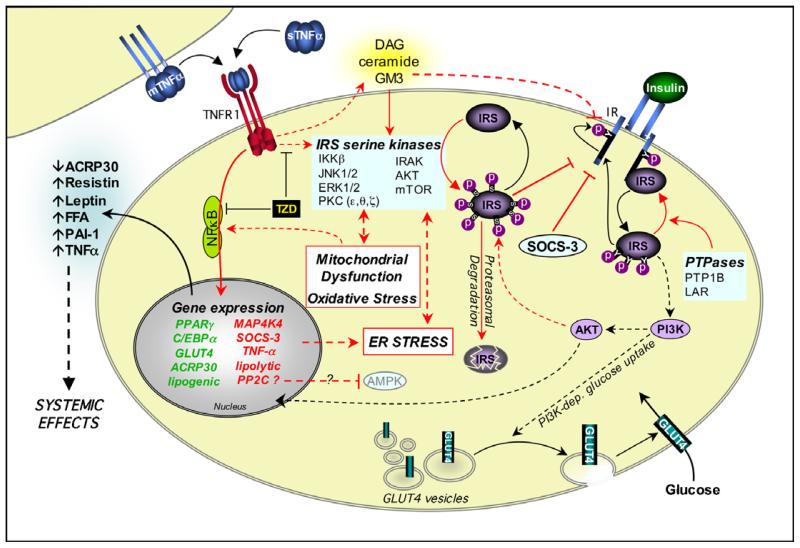
Fig. 2. Mechanisms of TNF-α-induced insulin resistance in adipose tissue.
Insulin mediates metabolic effects by binding to the insulin receptor (IR). The IR has intrinsic tyrosine kinase activity, and insulin binding promotes autophosphorylation of the IR on key intracellular tyrosines. This allows the recruitment of insulin receptor substrates (IRS) such as IRS-1. IR-bound IRS proteins are themselves phosphorylated on tyrosine residues, allowing the activation of signalling pathways such as phosphatidylinositol 3-kinase (PI3K) and AKT. These exert downstream effects such as translocation of GLUT4 from intracellular storage vesicles to the plasma membrane. Protein tyrosine phosphatases (PTPases) can abrogate IR-proximal signalling. TNF-α also impairs IR-proximal signalling through the activation of IRS serine kinases and the production of toxic lipids, as described in Figure 1. TNF-α further compromises insulin action through transcriptional effects, possibly mediated via NFκB activation. These cause downregulation of components required for insulin responsiveness and upregulation of factors that may further impair both local and systemic insulin sensitivity, such as SOCS-3. Transcriptional effects may also promote cellular stresses that can impair insulin signalling. Thiazolidinediones (TZD) impair TNF-α-induced insulin resistance. Black arrows indicate effects that promote insulin signalling. Red arrows indicate effects that antagonise insulin signalling. Effects on gene expression are indicated as described in Fig. 1.