Table 1.
Activity and Solubility of Test Compounds
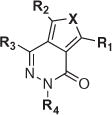
|
Solubility in assay buffer | K18PL ThT assay | K18PL centrifugation assay | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Compd number | R1 | R2 | R3 | R4 | X | Upper solubility limit (μM) | IC50 (μM) | Max % inhib. | % Soluble |
| 26 | −NH2 | −H | −COOEt | 4-F-Ph | S | >200 | 1.0 ± 1.11 | 77 | 63 |
| a29 | −NH | −H | −COOEt | 4-Cl-Ph | S | 13 | 4.0 ± 2.99b | 71 | 47 |
| 27 | −NH2 | −H | −COOEt | 2-Cl-Ph | S | 12.8 | 2.0 ± 1.42b | 75 | 71 |
| 28 | −NH2 | −H | −COOEt | 3-Cl-Ph | S | 32 | 3.2 ± 2.02b | 82 | 73 |
| 30 | −NH2 | −H | −COOEt | 4-CF3-Ph | S | 32 | DNC | 48 | 27 |
| 31 | −NH2 | −H | −COOEt | 4-OCF3-Ph | S | 12.8 | DNCb | 41 | 29 |
| 32 | −NH2 | −H | −COOEt | 4-OH-Ph | S | 12.8 | 5.0 ± 1.29 | 81 | 69 |
| 33 | −NH2 | −H | −COOEt | 4-iPr-Ph | S | 32 | DNCb | 8 | 17 |
| 66 | −NHCH3 | −H | −COOEt | 4-Cl-Ph | S | 80 | DNC | 39 | 51 |
| 67 | −NH2 | −Cl | −COOEt | 4-Cl-Ph | S | 32 | 2.0 ± 1.53 | 88 | 65 |
| 68 | −NH2 | −Br | −COOEt | 4-Cl-Ph | S | 12.8 | 4.0 ± 1.10 | 92 | 62 |
| 69 | −OH | −OH | −COOEt | 4-Cl-Ph | NH | >200 | DNC | 57 | 15 |
| 34 | −NH2 | −H | −COOH | 4-F-Ph | S | >200 | 2.0 ± 1.27 | 84 | 83 |
| a37 | −NH | −H | −COOH | 4-Cl-Ph | S | >200 | 5.0 ± 2.15b | 84 | 74 |
| 35 | −NH2 | −H | −COOH | 2-Cl-Ph | S | >200 | 3.2 ± 1.58 | 86 | 64 |
| 36 | −NH2 | −H | −COOH | 3-Cl-Ph | S | 32 | 1.6 ± 1.22 | 92 | 73 |
| 38 | −NH2 | −H | −COOH | 4-CF3-Ph | S | >200 | 19.9 ± 1.10 | 88 | 83 |
| 39 | −NH2 | −H | −COOH | 4-OCF3-Ph | S | >200 | 1.6 ± 1.53 | 92 | 71 |
| 40 | −NH2 | −H | −COOH | 4-OH-Ph | S | >200 | 4.0 ± 1.58b | 84 | 70 |
| 70 | −OH | −H | −COOH | 4-F-Ph | S | >200 | 2.5 ± 1.26b | 92 | 87 |
| 42 | −NH2 | −H | −CONH-iPr | 4-F-Ph | S | >200 | 1.6 ± 1.39 | 89 | 77 |
| 43 | −NH2 | −H | −CONH-cPr | 4-F-Ph | S | >200 | 2.5 ± 2.93b | 88 | 78 |
| 44 | −NH2 | −H | −CONH–CH2CF3 | 4-F-Ph | S | >200 | 8.0 ± 1.28 | 83 | 75 |
| a1 | −NH2 | −H | −CONH-cPr | 4-Cl-Ph | S | 32 | 8.0 ± 2.48b | 83 | 69 |
| 45 | −NH2 | −H | −CONH-cPr | 2-Cl-Ph | S | 80 | DNC | 68 | 44 |
| 46 | −NH2 | −H | −CONH-iPr | 3-Cl-Ph | S | 32 | 3.2 ± 2.02b | 82 | 73 |
| 47 | −NH2 | −H | −CONH-cPr | 4-CF3-Ph | S | 80 | 6.3 ± 2.28 | 64 | 83 |
| 48 | −NH2 | −H | −CONH-iPr | 4-OCF3-Ph | S | 80 | 15.8 ± 1.95 | 67 | 42 |
| 49 | −NH2 | −H | −CONH-iPr | 4-OH-Ph | S | >200 | 15.8 ± 1.38 | 86 | 84 |
| 50 | −NH2 | −H | −CONH-cPr | 4-iPr-Ph | S | 80 | 4.0 ± 2.88 | 82 | 86 |
| 72 | −OH | −H | −CONH-iPr | 4-Cl-Ph | S | ND | 10.0 ± 1.25 | 96 | 85 |
| 55 | −NH2 | −H | −CH3 | −H | S | >200 | 8.0 ± 1.87b | 87 | 81 |
| 56 | −NH2 | −H | −CH3 | −CH3 | S | >200 | 2.5 ± 1.19 | 82 | 76 |
| 57 | −NH2 | −H | −CH3 | iPr | S | 80 | 19.9 ± 1.16 | 95 | 17 |
| 58 | −NH2 | −H | −CH3 | CH2CF3 | S | >200 | 10± 1.18b | 81 | 78 |
| 59 | −NH2 | −H | −CH3 | Cyclohexyl | S | >200 | 31.6 ± 1.12 | 65 | 86 |
| 60 | −NH2 | −H | −CH3 | 2-Py | S | >200 | 2.0 ± 1.27 | 85 | 78 |
| 61 | −NH2 | −H | −CH3 | 3-Py | S | >200 | 4.0 ± 1.41 | 74 | 84 |
| 62 | −NH2 | −H | −CH3 | −Bn | S | 80 | 1.6 ± 1.24 | 89 | 73 |
| 63 | −NH2 | −H | −CH3 | 4-Cl-Ph | S | 80 | 2.0 ± 1.23 | 85 | 70 |
| 64 | −NH2 | −H | −Ph | CH2CF3 | S | >200 | 6.3 ± 1.63b | 103 | 82 |
| 65 | −NH2 | −H | −Ph | iPr | S | >200 | 19.9 ± 1.63b | 89 | 92 |
| 21 |
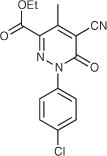
|
12.8 | DNCb | 4.4 | 21 | ||||
| 54 |
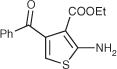
|
>200 | DNC | 29 | 6.5 | ||||
Previously reported data;7 DNC = did not calculate due to absence of asymptote; ND = not determined; all IC50 values were determined through sigmoidal curve fitting of compound concentration-response analyses, with each concentration tested in triplicate.
All results are from a single curve fit, except where indicated by, where the mean of 2 or more separate analyses are reported.
