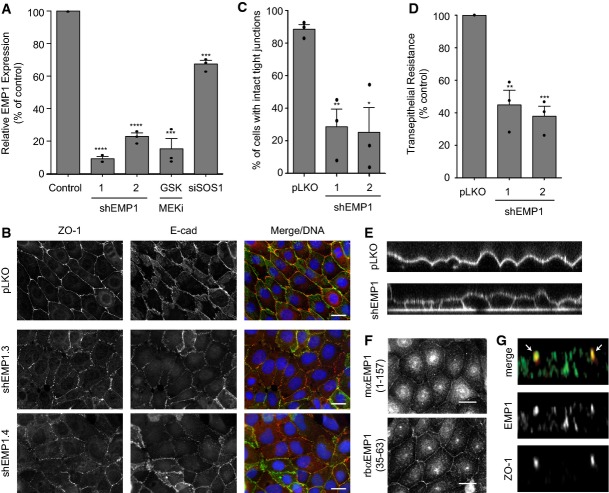
Figure 4. EMP1 is required for bronchial epithelial tight junction formation and function.
- 16HBE cells were stably infected with lentiviral vector pLKO alone or expressing EMP1 shRNAs (1 or 2). Total RNA was isolated and analysed for EMP1 expression using TaqMan/qPCR with a GAPDH control. Wild-type cells treated with 500 nM GSK1120212 or siSOS1 for 4 days were also analysed. Error bars denote mean ± SEM, and dots indicate individual data points. ***P < 0.0002 (GSK = 0.0002, siSOS1 = 0.0001); ****P < 0.0001.
- Cells as in (A) were fixed and stained for ZO-1, E-cadherin and DNA. Scale bar, 20 μm.
- Quantification of tight junction phenotype of cells as in (A). > 500 cells were counted per sample/experiment, across n = 3 independent experiments (dots indicate individual data points). Error bars denote mean ± SEM. *P = 0.0148; **P = 0.006.
- Transepithelial resistance (TER) was measured in 16HBE cells as in (A) on day 4 post-seeding. Error bars denote mean ± SEM, and dots indicate individual data points. **P = 0.0036; ***P = 0.0005.
- Cells as in (A) were seeded on glass-bottomed dishes for 4 days. FM 4–64 dye was applied to the media and confocal z-stacks acquired.
- 16HBE cells were fixed and stained for endogenous EMP1 and DNA using two different commercial antibodies. Scale bar, 20 μm.
- 16HBE cells were seeded on glass coverslips for 10 days to form a mature, polarised monolayer. Cells were fixed and costained for EMP1 and ZO-1 and then analysed by confocal microscopy. A representative z-stack is presented; arrows indicate ZO-1-positive tight junctions.
Data information: All data are representative of n = 3 independent experiments.