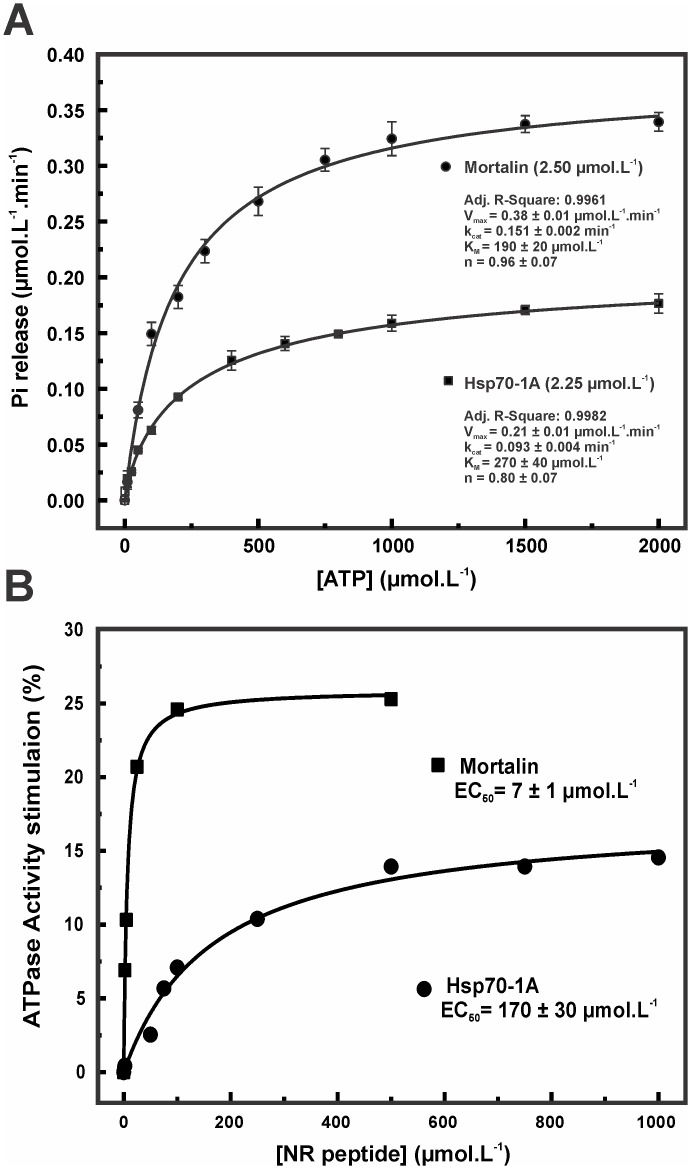
Fig 4. Mortalin has higher ATPase activity than Hsp70–1A.
A) Mortalin (2.50 μmol.L-1) and Hsp70–1A (2.25 μmol.L-1) were incubated with ATP (0–2 mmol.L-1) for 90 min at 37°C, and the Pi released as a result of ATP hydrolysis was quantified. The data were treated through Michaelis-Menten fitting for determination of the kinetic parameters, which are presented in the Figure and Table 2. The results suggested that both mortalin and Hsp70–1A exhibit low ATPase activity. Despite these findings, based on the kcat value, mortalin presented higher ATPase activity than Hsp70–1A, although the KM values of both are similar. B) Relative ATPase activity stimulation. Effect of the NR peptide titration on the basal ATPase activity of mortalin and Hsp70–1A at 1 mmol.L-1 ATP.