Abstract
Seabirds have been identified and used as indicators of ecosystem processes such as climate change and human activity in nearshore ecosystems around the globe. Temporal and spatial trends have been documented at large spatial scales, but few studies have examined more localized patterns of spatiotemporal variation, by species or functional group. In this paper, we apply spatial occupancy models to assess the spatial patchiness and interannual trends of 18 seabird species in the Puget Sound region (Washington State, USA). Our dataset, the Puget Sound Seabird Survey of the Seattle Audubon Society, is unique in that it represents a seven-year study, collected with a focus on winter months (October–April). Despite historic declines of seabirds in the region over the last 50 years, results from our study are optimistic, suggesting increases in probabilities of occurrence for 14 of the 18 species included. We found support for declines in occurrence for white-winged scoters, brants, and 2 species of grebes. The decline of Western grebes in particular is troubling, but in agreement with other recent studies that have shown support for a range shift south in recent years, to the southern end of California Current.
Keywords: Puget Sound, Seabirds, Citizen-science, Hotspots, Spatial models, Occupancy models, Salish Sea
Introduction
Ecologists and conservation practitioners have long focused on describing species distribution and estimating changes in abundance (Holmes, 2001) or occurrence through time (MacKenzie et al., 2006). Using species distribution modeling to identify the spatial variability and hotspots of a species’ distribution has multiple implications for science and management. From a conservation perspective, incorporating spatial variation in models may assist in selecting areas to protect or predicting where species are likely to persist (Cabeza & Moilanen, 2001; Naujokaitis-Lewis et al., 2009). From a theoretical ecology perspective, null or neutral models of species’ occurrence may be useful in predicting species diversity or community assembly (Gotelli, 2000; Gotelli & McGill, 2006). Finally, the inclusion of spatial variation has implications for management and policy in that accounting for spatial heterogeneity may help in forecasting how species may respond to future environmental conditions, such as range shifts in response to climate change (Jetz, Wilcove & Dobson, 2007).
In addition to quantifying spatial variation, species distribution modeling can be used to assess temporal trends in occurrence, which themselves may be spatially structured as well. Mechanisms responsible for spatially structured trends may include changing habitat conditions, behavior, or prey availability (Ward et al., 2010). Spatially structured anthropogenic disturbances (e.g., wildfires, oil spills, climate change, urbanization) may have similar impacts, and collectively ignoring such underlying spatial variation when it exists may lead to poor estimation of temporal trends (Hoeting et al., 2006).
Models that incorporate both spatial and temporal variation represent a rapidly evolving field in ecology (Hooten & Wikle, 2008; Latimer et al., 2006; Shelton et al., 2014). While many of these methods have been in the statistical literature for decades (Banerjee, Gelfand & Carlin, 2005; Cressie & Wikle, 2011), ecological data often present a unique set of challenges relative to data from other fields. Compared to other disciplines, ecological data on species abundance is often corrupted by observation error, which represents uncertainty arising from taking measurements or sampling a fraction of the population (Holmes, 2001). Similarly, in conducting studies of species presence–absence, detections may be missed, resulting in false-negatives (MacKenzie et al., 2006). Because of recent computational advances, statistical models that include both spatial and temporal variation are now widely available to ecologists and offer a powerful tool for assessing changes in species distributions through time.
As data hungry methods have advanced, monitoring programs have suffered in recent years because of declining budgets and an increased need for cost efficient survey techniques. In the face of recent reductions in monitoring programs, one potentially underutilized resource is citizen science, which may be a useful tool for conducting baseline environmental monitoring or helping to inform management actions or restoration activities (Cooper et al., 2007). Participation in these volunteer-based programs appears to have increased in recent years (Silvertown, 2009). Some of the longest running citizen-science programs in North America are related to bird watching. Large-scale volunteer programs like the Audubon Christmas Bird Count and the North American Breeding Bird Survey (BBS) have been effective at collecting vast amounts of survey data on commonly occurring bird species (Sauer et al., 2014). The strength of these programs is their duration, large spatial extent, and consistent methodologies over time, enabling them to be useful in monitoring species assemblages and distribution shifts in response to changing climate (Hitch & Leberg, 2007).
In the Pacific Northwest, similar regional-scale citizen-science programs focused on seabirds have been established. These include the Coastal Observation and Seabird Survey Team (COASST) (Hamel et al., 2009; Litle, Parrish & Dolliver, 2007; Parrish et al., 2007) and the British Columbia Coastal Waterbird Survey (Crewe et al., 2012), which have also been developed to address conservation questions and establish baseline monitoring. These regional citizen-science programs help inform restoration actions in Puget Sound (Washington State), where one of the largest ecosystem restoration programs in the nation is underway (Puget Sound Partnership, 2014). The Puget Sound ecosystem is part of the Salish Sea (which also includes the Strait of Juan de Fuca and the Strait of Georgia), and has been affected by widespread environmental degradation largely associated with increased urbanization (effects summarized in Puget Sound Partnership, 2009; Ruckelshaus & McClure, 2007). Puget Sound consists of over 4,000 km of coastline, with a suite of high-value ecosystem services, including commercial fisheries and various recreation opportunities (e.g., Tallis & Polasky, 2009). A portfolio of ecosystem indicators has been developed and incorporated into restoration goals for the Puget Sound region to monitor ecological conditions, including seabirds (Kershner et al., 2011; Puget Sound Partnership, 2013).
A limitation of using the data from many citizen-science programs—including those from regional seabird monitoring programs in the Pacific Northwest—has been that survey effort is generally not quantified. A limited number of agency-funded seabird surveys have been conducted that allow the assessment of trends. To assess winter seabird densities, the Washington Department of Fish and Wildlife (WDFW) has conducted annual aerial surveys since 1992, representing a 6-week snapshot of density (Anderson et al., 2009; Nysewander et al., 2005). These annual transects occur in 13–18% of the nearshore (<20 m depth) and 3–6% of the offshore (>20 m depth) marine waters in Puget Sound, ranging from southern Puget Sound to the Canadian border. Results from the WDFW aerial seabird surveys suggested that the density of some species, including Western Grebes (Aechmophorus occidentalis), has declined over the last two decades (Bower, 2009; Evenson, 2010; Vilchis et al., 2014). However, the cause(s) of these declines and the effects of environmental drivers on seabird density remain largely unknown. To complement the WDFW seabird survey both spatially and temporally, and to establish further baseline monitoring of local seabird species occurrence and abundance in winter months, the Seattle Audubon Society initiated the shore-based Puget Sound Seabird Survey (PSSS) in 2007. This program is unique in Puget Sound, in that volunteers monitor study sites in nearshore habitats monthly, from late fall to early spring (October–April). The October–April period was chosen because this is the window of greatest seabird abundance and diversity for this ecosystem. This survey also represents a good example of a scientifically rigorous citizen-science effort because survey effort is quantified and volunteers are trained annually and are the subject of ongoing validation studies to quantify biases, such as species misidentification.
Recent research has demonstrated that rigorous statistical models can be applied to volunteer-based surveys, yielding a relatively large impact, particularly when agency or industry-led data collection efforts are limited (Thorson et al., 2014). The primary objective of our analysis was to apply spatiotemporal models to data from the Puget Sound Seabird Survey to (1) evaluate relative hotspots of occurrence over the period 2007–2014, (2) evaluate temporal trends in occupancy over this period, and (3) establish a baseline for future monitoring in the region. These spatial and temporal estimates of occupancy may also be useful to refine the list of indicator species used to quantify ecosystem change or restoration progress.
Methods
PSS survey data collection
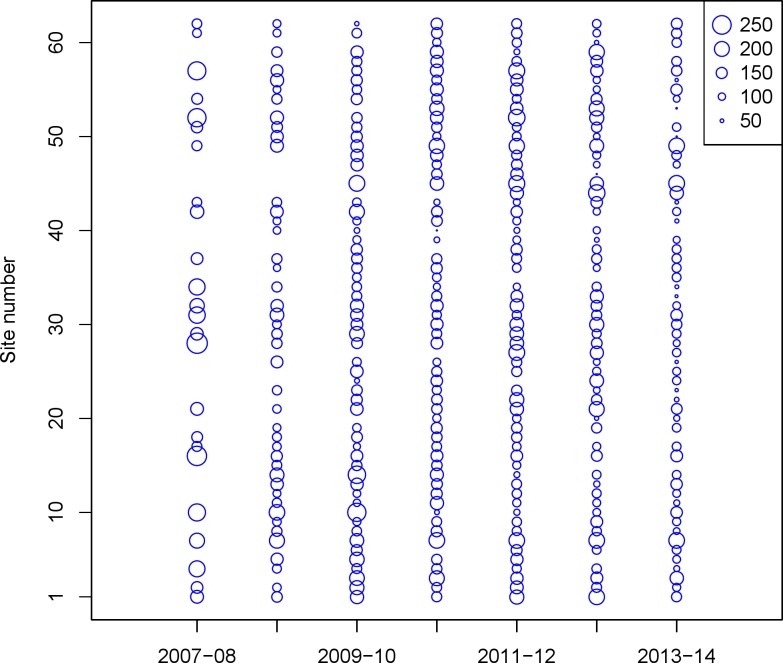
Beginning in October 2007, teams of 2–4 volunteer birdwatchers were trained by Seattle Audubon staff to collect data on birds in the nearshore environment of Puget Sound. Though the species encountered includes waterfowl, we collectively refer to all species as ‘seabirds.’ Each observer team was responsible for monthly surveys (October–April) at selected sites. Many of the seabird species in the region overwinter in Puget Sound, and are of highest abundance in late fall—early spring. The PSSS survey sites were selected non-randomly due to dependence on public access (parks, beach access), but they were selected to be spaced at least 1.6 km apart. Observer teams recorded all species present out to 300 m from shore for a minimum of 15 min, but some site visits lasted up to 60 min. To minimize the variability of weather conditions, tidal stage, and the risk of double counting birds at multiple survey sites, volunteer teams completed their monthly surveys on the same date within a specific four-hour window (two hours on either side of daylight high tide) on the first Saturday of each month. In each subsequent year of surveys, we added sites to cover parts of northern and southern Puget Sound. For this study, we limited our analysis to 62 sites with at least 15 visits (Fig. 1; Table 1). Additional details on the survey and monitoring, as well as additional maps can be found on the PSSS website, www.seabirdsurvey.org.
Figure 1.
Minutes of sampling effort recorded (across all observer pairs and months in the Puget Sound Seabird Survey that are included in our analysis), 2007–2014.
Table 1.
Name, latitude, and longitude of the 62 sites included in our analysis.
| Site | Lat (°N) | Lon (°W) | Site | Lat (°N) | Lon (°W) |
|---|---|---|---|---|---|
| 1. 60th St Viewpoint | 47.6723 | 122.4062 | 32. Mee Kwa Mooks | 47.5637 | 122.4070 |
| 2. Alki Beach | 47.5784 | 122.4144 | 33. Mukilteo State Park | 47.9478 | 122.3071 |
| 3. Boston Harbor | 47.1396 | 122.9029 | 34. Myrtle Edwards Park | 47.6268 | 122.3775 |
| 4. Brace Point | 47.5152 | 122.3964 | 35. Narrows Park | 47.2671 | 122.5641 |
| 5. Brown’s Point | 47.3058 | 122.4443 | 36. Normandy Beach Park | 47.4116 | 122.3401 |
| 6. Burfoot County Park | 47.1310 | 122.9046 | 37. North Redondo Boardwalk | 47.3507 | 122.3238 |
| 7. Carkeek Park | 47.7125 | 122.3796 | 38. Olympia waterfront | 47.0582 | 122.9020 |
| 8. Cromwell East | 47.2709 | 122.6110 | 39, Owens Beach Pt Defiance | 47.3128 | 122.5280 |
| 9. Cromwell West | 47.2714 | 122.6191 | 40. Penn Cove Pier | 48.2228 | 122.6883 |
| 10. Dash Pt State Park | 47.3204 | 122.4141 | 41. Penrose State Park | 47.2601 | 122.7450 |
| 11. DeMolay Boys Camp (E) | 47.2777 | 122.6662 | 42. Pier 57 | 47.6062 | 122.3429 |
| 12. DeMolay Boys Camp (W) | 47.2775 | 122.6668 | 43. Pier 70 | 47.6149 | 122.3573 |
| 13. Discovery Park West | 47.6674 | 122.4227 | 44. Point No Point | 47.9122 | 122.5265 |
| 14. Dumas Bay Park | 47.3263 | 122.3853 | 45. Pt Wilson | 48.1441 | 122.7538 |
| 15. Duwamish Head | 47.5954 | 122.3876 | 46. Purdy Spit South | 47.3817 | 122.6348 |
| 16. Edmonds north | 47.8114 | 122.3891 | 47. Raft Island north | 47.3318 | 122.6700 |
| 17. Edmonds south | 47.8033 | 122.3947 | 48. Raft Island south | 47.3261 | 122.6675 |
| 18. Elliott Bay Water Taxi Pier | 47.5898 | 122.3800 | 49. Richmond Beach | 47.7636 | 122.3858 |
| 19. Fox Island Fishing Pier | 47.2287 | 122.5898 | 50. Ruston Way | 47.2948 | 122.4990 |
| 20. Frye Cove County Park | 47.1152 | 122.9643 | 51. Saltwater State Park | 47.3728 | 122.3249 |
| 21. Golden Gardens | 47.6928 | 122.4056 | 52. Seahurst Park | 47.4781 | 122.3638 |
| 22. Howarth State Park | 47.9642 | 122.2407 | 53. Sinclair Inlet | 47.5398 | 122.6621 |
| 23. Jack Hyde Park | 47.2758 | 122.4622 | 54. South Redondo Boardwalk | 47.3434 | 122.3328 |
| 24. Kayak Point State Park | 48.1373 | 122.3668 | 55. The Cove | 47.4428 | 122.3563 |
| 25. Kopachuck | 47.3101 | 122.6874 | 56. Thea’s Park | 47.2620 | 122.4398 |
| 26. Les Davis Pier | 47.2836 | 122.4813 | 57. Three Tree Point | 47.4522 | 122.3792 |
| 27. Libbey Beach County Park | 48.2322 | 122.7668 | 58. Titlow Beach | 47.2469 | 122.5536 |
| 28. Lincoln Park | 47.5263 | 122.3949 | 59. Tolmie State Park | 47.1209 | 122.7761 |
| 29. Lowman Park | 47.5403 | 122.3974 | 60. Totten Inlet | 47.1540 | 122.9645 |
| 30. Luhr Beach | 47.1008 | 122.7272 | 61. West Point north | 47.6624 | 122.4335 |
| 31. Magnolia Bluff | 47.6313 | 122.3954 | 62. West Point south | 47.6610 | 122.4330 |
Species selection
Over the first seven years of the PSSS (the most recent ending in spring 2014), observer teams recorded 75 unique seabird species. While many of these species may be useful as indicators of various ecosystem processes or human impacts, we focused our analysis on 18 species that have previously been identified as useful seabird indicator species in the region (Table 2; Pearson & Hamel, 2013) because of their relative abundance and dependence on the marine waters of the Puget Sound (Gaydos & Pearson, 2011). These species can be aggregated into five distinct groups: alcids, cormorants, grebes, loons, and waterfowl. Some of the species breed locally in Puget Sound, while others are transient in the Sound, breeding elsewhere (Table 2). Similarly, the species represent a range of diets and behaviors (Pearson & Hamel, 2013), from piscivores (alcids, loons, grebes, cormorants) to omnivores (seaducks and other waterfowl).
Table 2.
The 18 species included in our analysis of the Puget Sound Seabird Survey. Rows in bold represent species that breed locally (in Puget Sound).
| Common name | Scientific name | Group |
|---|---|---|
| Common murre | Uria aalge | Alcids |
| Marbled murrelet | Brachyramphus marmoratus | Alcids |
| Pigeon guillemot | Cepphus columba | Alcids |
| Rhinoceros auklet | Cerorhinca monocerata | Alcids |
| Brandt’s cormorant | Phalacrocorax penicillatus | Cormorants |
| Pelagic cormorant | Phalacrocorax pelagicus | Cormorants |
| Horned grebe | Podiceps auritus | Grebes |
| Red-necked grebe | Podiceps grisegena | Grebes |
| Western grebe | Aechmophorus occidentalis | Grebes |
| Common loon | Gavia immer | Loons |
| Pacific loon | Gavia pacifica | Loons |
| Red-throated loon | Gavia stellata | Loons |
| Brant | Branta bernicla | Waterfowl |
| Bufflehead | Bucephala albeola | Waterfowl |
| Common goldeneye | Bucephala clangula | Waterfowl |
| Harlequin duck | Histrionicus histrionicus | Waterfowl |
| Surf scoter | Melanitta perspicillata | Waterfowl |
| White-winged scoter | Melanitta deglandi | Waterfowl |
Statistical modeling
For each species, we constructed matrices of presence–absence, dimensioned by the number of unique month-year combinations (t = 49) and sites (n = 62). Sites that were not visited during a given month were treated as NA values. We constructed a spatial occupancy model separately for each species to incorporate spatial patchiness as well as annual and seasonal variation. The model describing the probability of species presence can be represented as zi,j ∼ Ber(ϕi,j), where zi,j represents the unobserved presence–absence (1, 0), and logit(ϕi) = BXi + ETi + ε, where ϕi represents the site-specific occupancy probabilities at time i, Xi represents a matrix of covariates (Intercept, Month, Month2, Year, Y ear2), B represents a vector of estimated coefficients (shared across sites and time periods), E represents a linear offset coefficient for sampling effort (Ti), and ε represents a vector of spatially correlated random effects. We included time spent (Ti in minutes, ranging from 15 to 60) as a measure of effort to account for the higher chance of recording a species present during longer visits. The spatially correlated random effects are assumed to have the distribution ε ∼ MV N(0, Σ). For simplicity, we modeled the covariance matrix Σ as an exponential covariance function, Σi,j = σ2⋅Ii,j + τ⋅exp(−di,j/γ), where I represents an identity matrix, di,j is the Euclidian distance between sites i and j, and the scaling parameters (τ, γ) control how quickly covariance decays as a function of distance (Banerjee, Gelfand & Carlin, 2005; Ward et al., 2012). Our model could be modified to include more complex covariance functions (Cressie & Wikle, 2011) or spatial random effects that also vary temporally (Shelton et al., 2014). Because our model also includes an observation error component, however, we chose to make these spatial deviations temporally constant. The observation model, linking latent unobserved states (zi,j) to data (yi,j) can be written as yi,j|zi,j ∼ Ber(p⋅zi,j) (Royle & Kery, 2007), where p represents the probability of detection when a species is present.
All Markov Chain Monte Carlo (MCMC) estimation was conducted in R and JAGS (Plummer, 2003; R Development Core Team, 2014), using the R2jags package (Su & Yajima, 2014). We ran five parallel MCMC chains for each species, with a burn-in of 100,000 draws and additional sampling of 50,000 MCMC draws. Trace plots were used to visually assess convergence, and the Gelman–Rubin statistic (Gelman & Rubin, 1992) was used to quantify successful convergence. Not surprisingly, the only parameters that did not successfully converge (potential scale reduction factor >1.05) were several latent states (z) at sites that were not visited by observers in certain months. For the purposes of visualizing predicted hotspots of occupancy in Puget Sound, we used our model output to generate predictions (spatial maps, temporal trends) of species occupancy for a standardized 15 min survey. In addition to making these predictions for each of the 18 species included in our analysis, we attempted to identify hotspot areas, or the sites whose estimated occupancy probability was above the upper quartile (75%) across all sites. Finally, we generated specific occupancy probabilities for the five seabird groups: alcids, cormorants, loons, grebes, and waterfowl. For each group, the probability of occupancy for a group (corresponding to any species from that group being present) was calculated as .
Results
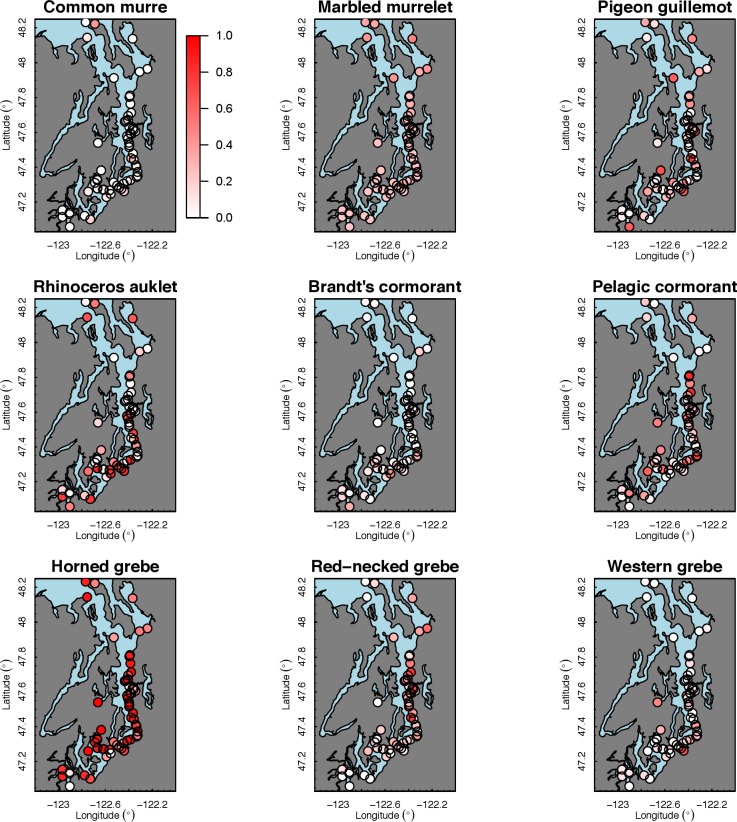
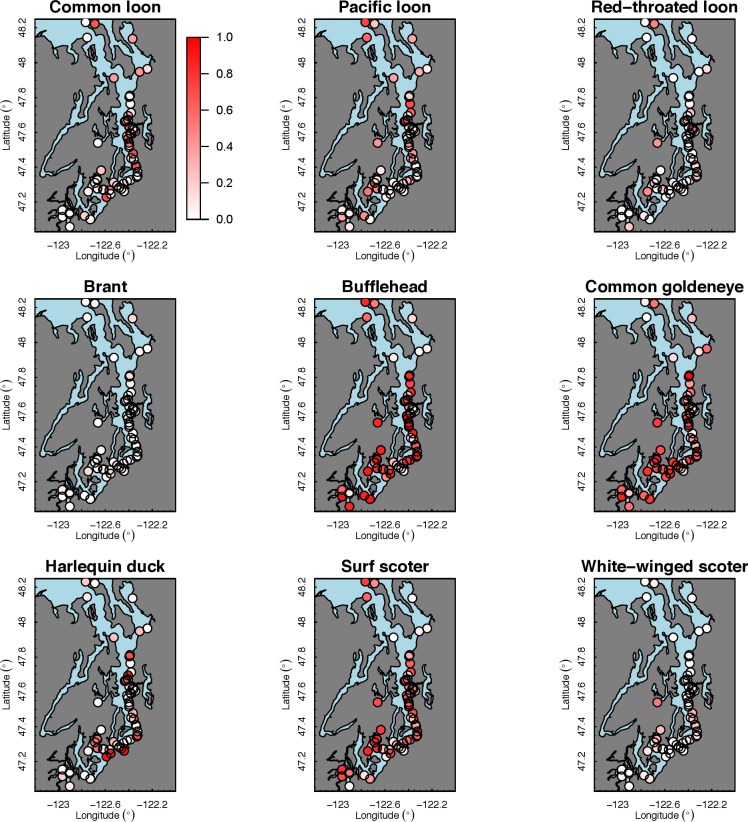
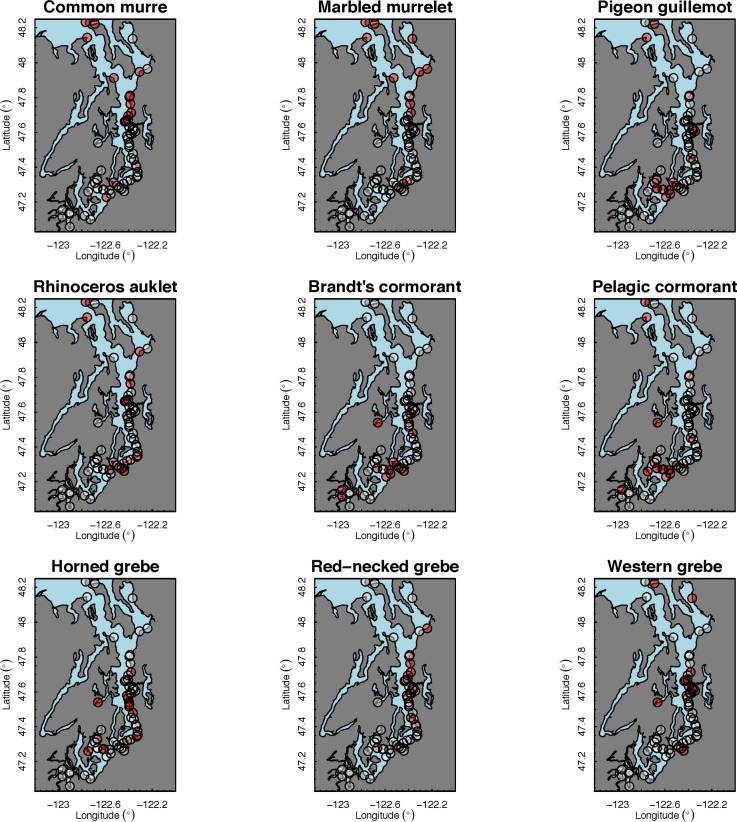
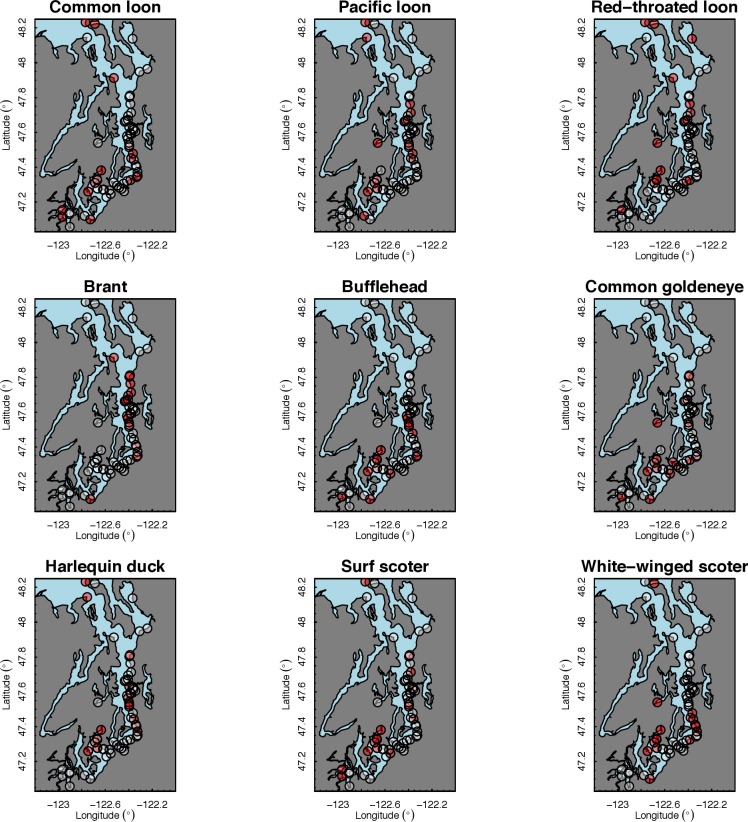
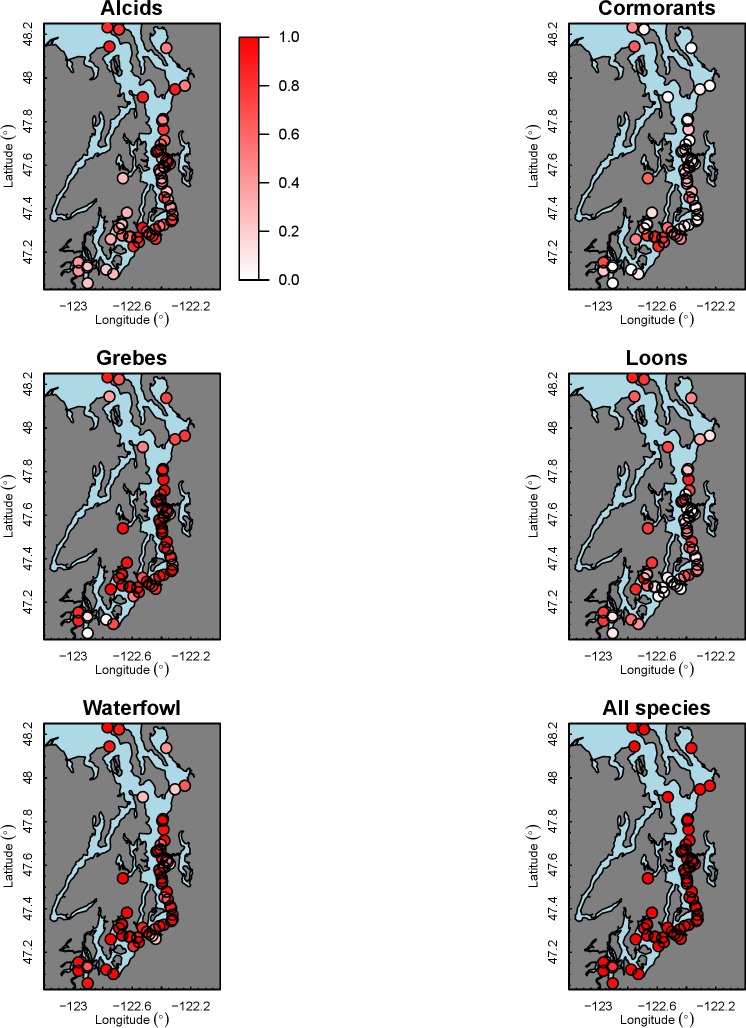
Our species occupancy maps reveal localized hotspots of occurrence in Puget Sound for some alcid and cormorant species (rhinoceros auklet, pelagic cormorant, Brandt’s cormorant (Fig. 2) as well as for loons and some waterfowl species like harlequin ducks (Fig. 3)). The individual species maps show that some species are ubiquitous in all nearshore habitat (horned grebes, goldeneyes, scoters), while others have a much more patchy distribution of occurrence (loons, rhinoceros auklets, pigeon guillemots). These patterns become even more apparent when we examine the sites in the upper quartile (75–100%) of the estimated occupancy probabilities across sites (Figs. 4 and 5). Some maps of very rare or very common species may not be informative, but areas of high bird density become more apparent when our estimated occupancy probabilities are calculated by group (Fig. 6). For example, each loon species in the survey is relatively rare (Fig. 3), but the aggregated spatial distribution of all loons shows several patches of high and low occurrence, with the highest density of occurrence in the central-south Puget Sound (Fig. 6).
Figure 2.
Estimated probability of occurrence for the 62 sites included in our analysis. Presented estimates are for alcids, cormorants, and grebes in December 2013. The color scale used to represent occurrence probabilities ranges from 0 (a species is not present) to 1 (occurrence is 100%).
Figure 3.
Estimated probability of occurrence for the 62 sites included in our analysis. Presented estimates are for loons and waterfowl in December 2013. The color scale used to represent occurrence probabilities ranges from 0 (a species is not present) to 1 (occurrence is 100%).
Figure 4.
Estimated hotspots of occurrence for the 62 sites included in our analysis, defined as probabilities in the upper quartile (75–100%) across sites (Fig. 2). Presented estimates are for alcids, cormorants, and grebes in December 2013. The color scale used to represent sites in the upper quartile is red (>75%) or white (<75%).
Figure 5.
Estimated hotspots of occurrence for the 62 sites included in our analysis, defined as probabilities in the upper quartile (75–100%) across sites (Fig. 2). Presented estimates are for loons and waterfowl in December 2013. The color scale used to represent sites in the upper quartile is red (>75%) or white (<75%).
Figure 6.
Aggregated probabilities of occurrence for each of the five groups in our analysis, as well as for all 18 species. For groups, these represent the probability of seeing any bird that is a member of that group; for all species, these represent the probability of seeing at least 1 bird (of the 18 species in our analysis). Estimates are shown for December 2013. The color scale used to represent occurrence probabilities ranges from 0 (not present) to 1 (occurrence is 100%).
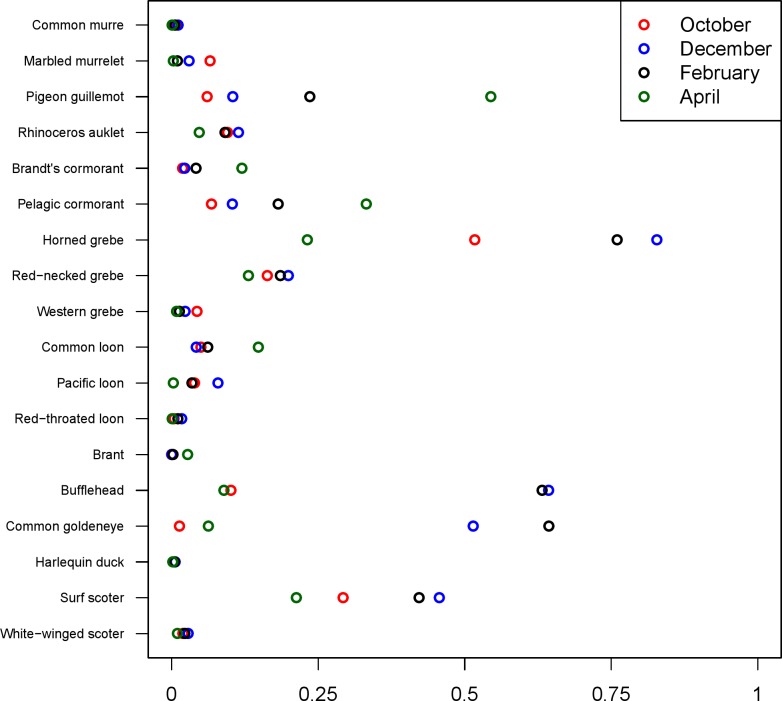
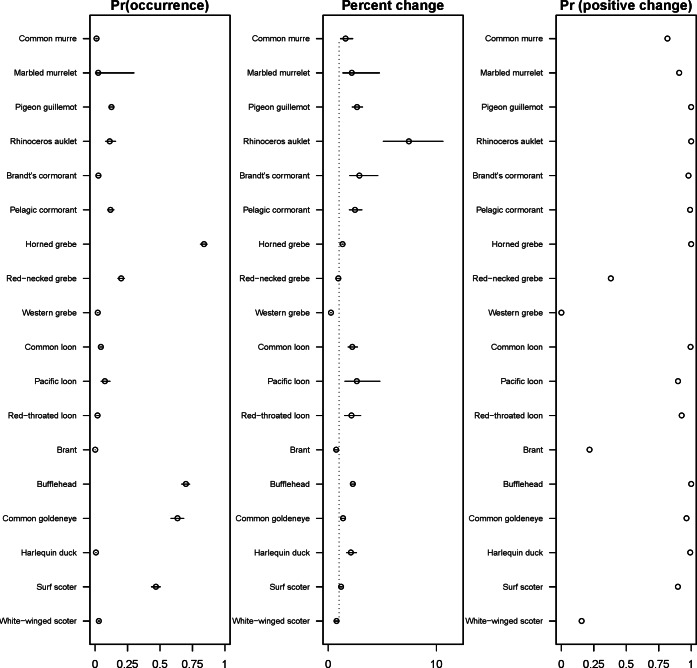
The 18 species included in our analysis showed a range of seasonal variation, with waterfowl species (bufflehead, common goldeneye, surf scoter) and grebes varying the most and peaking in December–January (Fig. 7). Although most of the 18 species had relatively small variation over the 7-month survey period, several species exhibited monotonic increases (pelagic cormorant, pigeon guillemot). Of the 18 species, the probabilities of trends in occurrence being positive over the 7-year survey were greater than 80% for 14 species (Fig. 8). Western grebes, white-winged scoters, and brants showed relatively strong negative trends in occurrence (probabilities of negative trends >99%, 84%, 79%, respectively).
Figure 7.
Estimated median probabilities of occurrence by month. Estimates are shown for the most recent year (October 2013–April 2014). Estimates for November, January, and March are not shown.
Figure 8.
Estimated probability of occurrence in the 2013–2014 seabird survey (with 25%, 50%, 75% intervals), percent change in the probability of occurrence from 2007 to 2013 (25%, 50%, 75% intervals), and the probability of the annual rate of change from 2007 to 2013 has been positive. All data (2007–2013) are used to estimate intra- and inter-annual trends.
The 18 species in our analysis represent a gradient of occurrence probabilities and trends over space. Several species from each group were relatively rare in central and south Puget Sound; the rarest species included two of the alcids (common murre, marbled murrelet), western grebes, all three loon species, and three of the waterfowl species (brant, harlequin duck, white-winged scoter; Figs. 2, 3 and 8). In contrast, horned grebes and three different waterfowl species (bufflehead, common goldeneye, surf scoter) were the most widely occurring (Fig. 8).
Discussion
Analyses that incorporate both spatial and temporal variation are becoming increasingly common in ecology. These types of analyses are widely applicable to virtually any type of observed data, from presence–absence to continuous observation measurements (Johnson et al., 2013; Shelton et al., 2014). Incorporating spatially structured random effects introduces a layer of statistical complexity that is warranted in many cases because predicted density estimates (both in space and time) are more precise (Thorson et al., in press).
Spatially-structured citizen-science datasets have been used at a large spatial scale, particularly in quantifying shifts in phenology linked to climate. One of the most frequently documented changes by citizen-science efforts has been shifts in breeding seasons (Hitch & Leberg, 2007; Hurlbert & Liang, 2012; Mayer, 2010). Spatially-structured statistical models have been fit to these types of datasets to improve estimates of trends (Hurlbert & Liang, 2012; Thorson et al., 2014), but few analyses have applied spatiotemporal models to data from citizen-science efforts to identify hotspots or areas of conservation concern at a fine spatial scale. Citizen-science programs, such as the Puget Sound Seabird Survey data analyzed here, offer a unique opportunity because both the temporal and spatial scales of data collection are much finer than national (Breeding Bird Survey) or regional (WA Department of Fish and Wildlife) efforts. If volunteer-driven science can result in relative indices of occurrence or abundance, it provides an extremely cost-effective approach for identifying local areas of risk (Hass, Hyman & Semmens, 2012) or potential hotspots of diversity that may be useful in conservation planning (e.g., establishing reserves) or permitting activities.
Using citizen-science data—either to complement existing datasets or to fill in data gaps when other surveys are absent—is particularly important for areas or habitats at risk. The PSSS may be a good model for adopting similar citizen-science efforts, either in other regions or for other applications that may also be used to study food webs—examples include monitoring water quality and the spread of invasive species (Silvertown, 2009). In addition to the historic decline of many seabird species (Bower, 2009), there are a number of other human impacts that have caused shifts or reorganization in the prey base (Blight et al., 2014) or competitors of seabirds (Harvey, Williams & Levin, 2012). These impacts could include effects of overfishing or bycatch (and associated impacts of derelict fishing gear; Good et al., 2009), climate change, toxins (Good et al., 2014), habitat loss (Raphael et al., in press), altered freshwater flow regimes, and the recovery of many top predators to historic levels (pinnipeds, harbor porpoise, bald eagles).
Although many seabird species in the Puget Sound region are thought to be depleted relative to abundances in the 1960s–1970s (Bower, 2009; Vilchis et al., 2014), our results present a more optimistic picture for a number of species over the last decade. Of the 18 species included in our analysis, we found strong support for 14 having increasing probabilities of occurrence, and these results are in agreement with recent studies in the region (for example, nesting surveys suggest Rhinoceros auklets are also increasing; Pearson et al., 2013). Many of the species that are occurring more frequently are those that breed in the region (Table 2). In the list of indicator species compiled by Pearson & Hamel (2013), some of these species (scoters, murrelets) were declining significantly when considering trends based on total abundance, so it is possible that species in decline have a less aggregated spatial distribution, resulting in their probability of detection increasing. Another possibility is that the PSS survey measures occurrence close to land, while trends from other surveys may represent slightly different habitats. Of the species not increasing, one species provided weak support for declining occurrence (white-winged scoter), and three species provided strong support for continued declines in occurrence (brant, western grebe, red-necked grebe). These three species in decline are also concerning because they are already rarely seen species in the PSSS data (Fig. 8).
There is no obvious mechanism for why the three declining species in our analysis exhibit a declining trend in occupancy, but some of these declines may be occurring at breeding colonies (not in Puget Sound) or resulting from shifts in prey abundance in the Puget Sound region. Some recent evidence suggests that there have been long-term changes in the base of the food web of the Salish Sea (Blight et al., 2014), and over-wintering seabird species that rely on forage fish are declining (Vilchis et al., 2014). Another mechanism that may also be related to shifts in the spatial distribution of prey is the large-scale shifts in seabird species’ ranges. For example, Wilson et al. (2013) used citizen-science data to show that western grebes appear to have shifted out of Puget Sound region to the southern end of the California Current. Our estimated declines in occupancy over the last seven years are largely in agreement with a continued decline in the occurrence of western grebes in the region. Like western grebes, brants and white-winged scoters over-winter in Puget Sound but breed elsewhere, and thus may be affected by threats in other ecosystems. Though the exact mechanisms responsible for these trends are not known, our trend estimates may be useful in prioritizing monitoring efforts or refining existing marine bird or ecosystem indicators in the region (Kershner et al., 2011; Pearson & Hamel, 2013).
Although the focus of our volunteer-driven surveys in the Puget Sound region are focused on identifying spatial hotspots and improving estimates of annual trends, citizen-science efforts like the PSSS may provide additional valuable baseline monitoring. The 7-year dataset analyzed here provides both a baseline for seabird monitoring in 2014, and also allows us to do a retrospective analysis of trends over this time period. For example, in the event of an oil spill in the region, PSSS data could provide 7 + years of pre-spill information on seabird distribution and abundance for comparison. Having a 7-year period as a baseline instead of just a single year is useful in that the year-to-year variability can be quantified. Such citizen-science efforts may also be scalable to different types of data collection that also involve spatially structured threats to marine ecosystems such as harmful algal blooms, ocean acidification, and fisheries activities.
Acknowledgments
The Puget Sound Seabird Survey was conceived, developed and implemented by the Seattle Audubon Society and continues to be a major focus of their science efforts. We thank the 250 + observers who braved wind and rain to collect bird data; this work would not be possible without their dedication. Thanks to J Smith, C Jordan, T Good, and two anonymous reviewers for providing helpful reviews of earlier drafts of this manuscript.
Funding Statement
Funding for the Puget Sound Seabird Survey was provided by Boeing, Sustainable Path Foundation, Russell Family Foundation, WDFW, and Patagonia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Eric J. Ward is an employee of NOAA, Kristin N. Marshall is a post-doc at NOAA, Scott F. Pearson is an employee of Washington Department of Fish and Wildlife, Nathalie Hamel is an employee of Puget Sound Partnership, Adam Sedgley and Toby Ross performed this work as employees of Seattle Audubon.
Author Contributions
Eric J. Ward and Kristin N. Marshall conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Toby Ross, Adam Sedgley, Todd Hass, Scott F. Pearson, Gerald Joyce, Nathalie J. Hamel, Peter J. Hodum and Rob Faucett conceived and designed the experiments, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
References
- Anderson et al. (2009).Anderson EM, Bowe JL, Nysewander DR, Evenson JR, Lovvorn JR. Changes in avifaunal abundance in a heavily-used wintering and migration site in Puget Sound, Washington during 1966–2007. Marine Ornithology. 2009;37:19–27. [Google Scholar]
- Banerjee, Gelfand & Carlin (2005).Banerjee S, Gelfand AE, Carlin BP. Hierarchical modeling and analysis for spatial data. Boca Raton: Chapman & Hall/CRC; 2005. [Google Scholar]
- Blight et al. (2014).Blight LK, Hobson KA, Kyser TK, Arcese P. Changing gull diet in a changing world: a 150-year stable isotope (δ13C, δ15N) record from feathers collected in the Pacific Northwest of North America. Global Change Biology. 2014 doi: 10.1111/gcb.12796. Epub ahead of print Dec 5 2014. [DOI] [PubMed] [Google Scholar]
- Bower (2009).Bower JL. Changes in marine bird abundance in the Salish Sea. Marine Ornithology. 2009;37:9–17. [Google Scholar]
- Cabeza & Moilanen (2001).Cabeza M, Moilanen A. Design of reserve networks and the persistence of biodiversity. Trends in Ecology & Evolution. 2001;16:242–248. doi: 10.1016/S0169-5347(01)02125-5. [DOI] [PubMed] [Google Scholar]
- Cooper et al. (2007).Cooper CB, Dickinson J, Phillips T, Bonney R. Citizen science as a tool for conservation in residential ecosystems. Ecology and Society. 2007;12(2):11. [Google Scholar]
- Cressie & Wikle (2011).Cressie N, Wikle CK. Statistics for spatio-temporal data. Hoboken: Wiley; 2011. [Google Scholar]
- Crewe et al. (2012).Crewe T, Barry K, Davidson P, Lepage D. Coastal waterbird population trends in the Strait of Georgia 1999–2011: results from the first 12 years of the British Columbia Coastal Waterbird Survey. British Columbia Birds. 2012;22:8–35. [Google Scholar]
- Evenson (2010).Evenson JR. Puget Sound assessment and monitoring program. Olympia: Washington Department of Fish and Wildlife; 2010. Analysis of marine bird data. [Google Scholar]
- Gaydos & Pearson (2011).Gaydos JK, Pearson SF. Birds and mammals that depend on the Salish Sea: a compilation. Northwest Naturalist. 2011;92:79–94. doi: 10.1898/10-04.1. [DOI] [Google Scholar]
- Gelman & Rubin (1992).Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Statistical Science. 1992;7:457–511. doi: 10.1214/ss/1177011136. [DOI] [Google Scholar]
- Good et al. (2009).Good TP, June JA, Etnier MA, Broadhurst MK. Ghosts of the Salish Sea: threats to marine birds in Puget Sound and the Northwest straits from derelict fishing gear. Marine Ornithology. 2009;37:67–76. [Google Scholar]
- Good et al. (2014).Good TP, Pearson SF, Hodum P, Boyd D, Anulacio BF, Ylitalo GM. Persistent organic pollutants in the diet of rhinoceros auklets (Cerorhinca monocerata) breeding in Puget Sound and the northern California Current. Marine Pollution Bulletin. 2014;86:367–378. doi: 10.1016/j.marpolbul.2014.06.042. [DOI] [PubMed] [Google Scholar]
- Gotelli (2000).Gotelli NJ. Null model analysis of species co-occurrence patterns. Ecology. 2000;81:2606–2621. doi: 10.1890/0012-9658(2000)081[2606:NMAOSC]2.0.CO;2. [DOI] [Google Scholar]
- Gotelli & McGill (2006).Gotelli NJ, McGill BJ. Null versus neutral models: what’s the difference. Ecography. 2006;29:793–800. doi: 10.1111/j.2006.0906-7590.04714.x. [DOI] [Google Scholar]
- Hamel et al. (2009).Hamel NJ, Burger AE, Charleton K, Davidson P, Lee S, Bertram DF, Parrish JK. Bycatch and beached birds: assessing mortality impacts in coastal net fisheries using marine bird strandings. Marine Ornithology. 2009;37:41–60. [Google Scholar]
- Hass, Hyman & Semmens (2012).Hass T, Hyman J, Semmens BX. Climate change, heightened hurricane activity, and extinction risk for an endangered tropical seabird, the black-capped petrel Pterodroma hasitata. Marine Ecology Progress Series. 2012;454:251–261. doi: 10.3354/meps09723. [DOI] [Google Scholar]
- Harvey, Williams & Levin (2012).Harvey CJ, Williams GD, Levin PS. Food web structure and trophic control in central Puget Sound. Estuaries and Coasts. 2012;35:821–838. doi: 10.1007/s12237-012-9483-1. [DOI] [Google Scholar]
- Hitch & Leberg (2007).Hitch AT, Leberg PL. Breeding distributions of North American bird species moving north as a result of climate change. Conservation Biology. 2007;21:534–539. doi: 10.1111/j.1523-1739.2006.00609.x. [DOI] [PubMed] [Google Scholar]
- Hoeting et al. (2006).Hoeting JA, Davis RA, Merton AA, Thompson SE. Model selection for geostatistical models. Ecological Applications. 2006;16(1):87–98. doi: 10.1890/04-0576. [DOI] [PubMed] [Google Scholar]
- Holmes (2001).Holmes EE. Estimating risks in declining populations with poor data. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5072–5077. doi: 10.1073/pnas.081055898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooten & Wikle (2008).Hooten MB, Wikle CK. A hierarchical Bayesian non-linear spatio-temporal model for the spread of invasive species with application to the Eurasian Collared-Dove. Environmental and Ecological Statistics. 2008;15:59–70. doi: 10.1007/s10651-007-0040-1. [DOI] [Google Scholar]
- Hurlbert & Liang (2012).Hurlbert AH, Liang ZF. Spatiotemporal variation in avian migration phenology: citizen science reveals effects of climate change. PLoS ONE. 2012;7(2):e31662. doi: 10.1371/journal.pone.0031662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetz, Wilcove & Dobson (2007).Jetz W, Wilcove DS, Dobson AP. Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biology. 2007;5:1211–1219. doi: 10.1371/journal.pbio.0050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson et al. (2013).Johnson DS, Conn PB, Hooten MB, Ray JC, Pond BA. Spatial occupancy models for large data sets. Ecology. 2013;94:801–808. doi: 10.1890/12-0564.1. [DOI] [Google Scholar]
- Kershner et al. (2011).Kershner J, Samhouri JF, James CA, Levin PS. Selecting indicator portfolios for marine species and food webs: a Puget Sound case study. PLoS ONE. 2011;6(10):e25248. doi: 10.1371/journal.pone.0025248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer et al. (2006).Latimer AM, Wu SS, Gelfand AE, Silander JA. Building statistical models to analyze species distributions. Ecological Applications. 2006;16:33–50. doi: 10.1890/04-0609. [DOI] [PubMed] [Google Scholar]
- Litle, Parrish & Dolliver (2007).Litle K, Parrish JK, Dolliver J. The coastal observation and seabird survey team—citizens monitoring coastal environmental health in Alaska. In: Brewer R, editor. Community-based coastal observing in Alaska: aleutian life forum 2006. Fairbanks: Alaska Sea Grant College Program; 2007. AK-SG-07-03, 21–38. [Google Scholar]
- MacKenzie et al. (2006).MacKenzie DI, Nichols JD, Royle JA, Pollock KH, Bailey LL, Hines JE. Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence. Burlington: Elsevier; 2006. [Google Scholar]
- Mayer (2010).Mayer A. Phenology and citizen science. BioScience. 2010;60:172–175. doi: 10.1525/bio.2010.60.3.3. [DOI] [Google Scholar]
- Naujokaitis-Lewis et al. (2009).Naujokaitis-Lewis IR, Curtis JMR, Arcese P, Rosenfeld J. Sensitivity analyses of spatial population viability analysis models for species at risk and habitat conservation planning. Conservation Biology. 2009;23:225–229. doi: 10.1111/j.1523-1739.2008.01066.x. [DOI] [PubMed] [Google Scholar]
- Nysewander et al. (2005).Nysewander DR, Evenson JR, Murphie BL, Cyra TA. Report of marine bird and marine mammal component, Puget Sound ambient monitoring program, for July 1992 to December1999. Olympia: Washington State Department of Fish and Wildlife; 2005. [Google Scholar]
- Parrish et al. (2007).Parrish JK, Bond N, Nevins H, Mantua N, Loeffel R, Peterson WT, Harvey JT. Beached birds and physical forcing in the California Current System. Marine Ecology-Progress Series. 2007;352:275–288. doi: 10.3354/meps07077. [DOI] [Google Scholar]
- Pearson & Hamel (2013).Pearson SF, Hamel NJ. Marine and terrestrial bird indicators for Puget Sound. Olympia: Washington Department of Fish and Wildlife and Puget Sound Partnership, 55; 2013. [Google Scholar]
- Pearson et al. (2013).Pearson SF, Hodum PJ, Good TP, Schrimpf M, Knapp SM. A model approach for estimating colony size, trends, and habitat associations of burrow-nesting seabirds. Condor. 2013;115:356–365. doi: 10.1525/cond.2013.110207. [DOI] [Google Scholar]
- Plummer (2003).Plummer M. JAGS: a program for analysis of Bayesian graphical models using Gibbs Sampling. Proceedings of the 3rd international workshop on distributed statistical computing; Vienna. 2003. Available at http://www.r-project.org/conferences/DSC-2003/Proceedings/Plummer.pdf . [Google Scholar]
- Puget Sound Partnership (2009).Puget Sound Partnership 2009. Puget Sound action agenda, protecting and restoring the Puget Sound ecosystem by 2020. Available at http://www.psp.wa.gov/downloads/AA2009/Action_Agenda_FINAL_063009.pdf .
- Puget Sound Partnership (2013).Puget Sound Partnership 2013. State of the Sound: A Biennial Report on the Recovery of Puget Sound. Tacoma: Puget Sound Partnership, 177. Available at http://www.psp.wa.gov/sos.php .
- Puget Sound Partnership (2014).Puget Sound Partnership 2014. The 2014/2015 action agenda for Puget Sound. Available at http://www.psp.wa.gov/2014_action_agenda_download.php .
- R Development Core Team (2014).R Development Core Team . R: a language and environment for statistical computing. Vienna: the R Foundation for Statistical Computing; 2014. Available at http://www.R-project.org/ [Google Scholar]
- Raphael et al. (in press).Raphael MG, Shirk AJ, Falxa GA, Pearson SF. Habitat associations of marbled murrelets during the nesting season in nearshore waters along the Washington to California coast. Journal of Marine Systems. 2014 In Press. [Google Scholar]
- Royle & Kery (2007).Royle JA, Kery M. A Bayesian state-space formulation of dynamic occupancy models. Ecology. 2007;88:1813–1823. doi: 10.1890/06-0669.1. [DOI] [PubMed] [Google Scholar]
- Ruckelshaus & McClure (2007).Ruckelshaus MH, McClure MM. Prepared in cooperation with the Sound Science collaborative team. Seattle: U.S. Dept. of Commerce, National Oceanic & Atmospheric Administration, Northwest Fisheries Science Center, 93; 2007. Sound science: synthesizing ecological and socioeconomic information about the puget sound ecosystem. Available at http://www.nwfsc.noaa.gov/research/shared/sound_science/documents/SoundScience07.pdf . [Google Scholar]
- Sauer et al. (2014).Sauer JR, Hines JE, Fallon JE, Pardieck KL, Ziolkowski DJJ, Link WA. The North American breeding bird survey, results and analysis 1966–2012. Version 02.19.2014. Laurel: USGS Patuxent Wildlife Research Center; 2014. [Google Scholar]
- Shelton et al. (2014).Shelton AO, Thorson JT, Ward EJ, Feist BE. Spatial, semi-parametric models improve estimates of species abundance and distribution. Canadian Journal of Fisheries and Aquatic Sciences. 2014;71(11):1655–1666. doi: 10.1139/cjfas-2013-0508. [DOI] [Google Scholar]
- Silvertown (2009).Silvertown J. A new dawn for citizen science. Trends in Ecology & Evolution. 2009;24:467–471. doi: 10.1016/j.tree.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Su & Yajima (2014).Su Y-S, Yajima M. R2jags: a package for running jags from R. (R package version 0.04-01) 2014 Available at http://CRAN.R-project.org/package=R2jags .
- Tallis & Polasky (2009).Tallis H, Polasky S. Mapping and valuing ecosystem services as an approach for conservation and natural-resource management. Annals of the New York Academy of Sciences. 2009;1162:265–283. doi: 10.1111/j.1749-6632.2009.04152.x. [DOI] [PubMed] [Google Scholar]
- Thorson et al. (2014).Thorson JT, Scheuerell MD, Semmens BX, Pattengill-Semmens CV. Demographic modeling of citizen science data informs habitat preferences and population dynamics of recovering fishes. Ecology. 2014;95:3251–3258. doi: 10.1890/13-2223.1. [DOI] [Google Scholar]
- Thorson et al. (in press).Thorson JT, Skaug H, Kristensen K, Shelton AO, Harms J, Benante J. The importance of spatial models for estimating the type and strength of density dependence. Ecological Applications. 2014 doi: 10.1890/14-0739.1. In Press. [DOI] [PubMed] [Google Scholar]
- Vilchis et al. (2014).Vilchis IL, Johnson CK, Evenson JR, Pearson SF, Barry KL, Davidson P, Raphael MG, Gaydos JK. Assessing ecological correlates of marine bird declines to inform marine conservation. Conservation Biology. 2014 doi: 10.1111/cobi.12378. Epub ahead of print Sept 5 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward et al. (2010).Ward EJ, Chirakkal H, Gonzalez-Suarez M, Aurioles-Gamboa D, Holmes EE, Gerber L. Inferring spatial structure from time-series data: using multivariate state-space models to detect metapopulation structure of California sea lions in the Gulf of California, Mexico. Journal of Applied Ecology. 2010;47:47–56. doi: 10.1111/j.1365-2664.2009.01745.x. [DOI] [Google Scholar]
- Ward et al. (2012).Ward EJ, Pess GR, Anlauf-Dunn K, Jordan CE. Applying time series models with spatial correlation to identify the scale of variation in habitat metrics related to threatened coho salmon (Oncorhynchus kisutch) in the Pacific Northwest. Canadian Journal of Fisheries and Aquatic Sciences. 2012;69:1773–1782. doi: 10.1139/f2012-096. [DOI] [Google Scholar]
- Wilson et al. (2013).Wilson S, Anderson EM, Wilson ASG, Bertram DF, Arcese P. Citizen science reveals an extensive shift in the winter distribution of migratory western grebes. PLoS ONE. 2013;8:e704. doi: 10.1371/journal.pone.0065408. [DOI] [PMC free article] [PubMed] [Google Scholar]