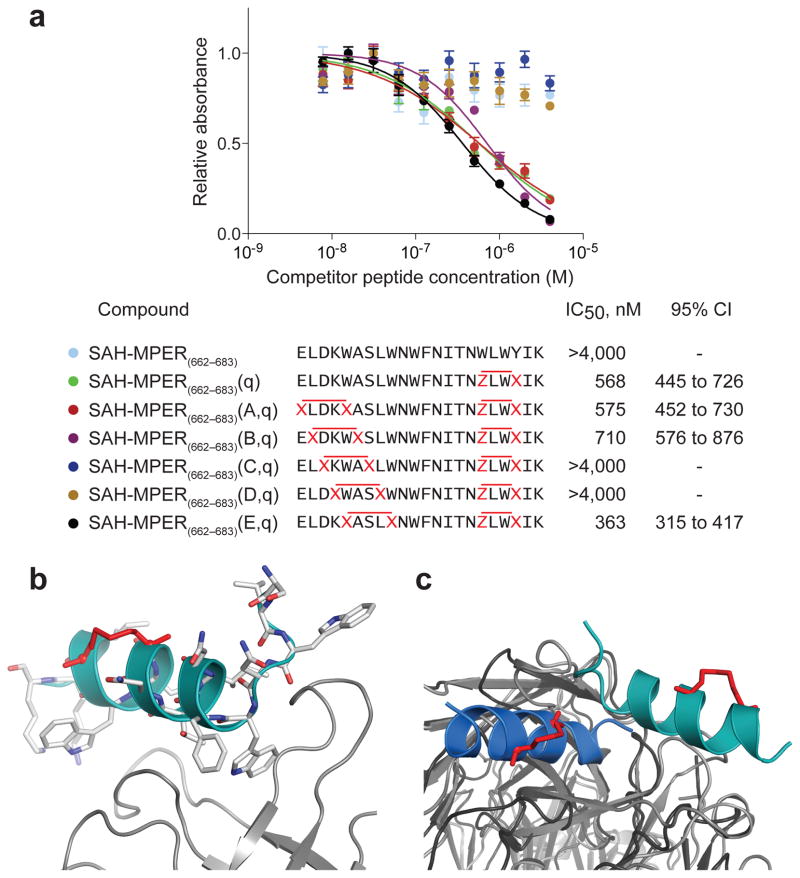
Figure 7. 10E8 Binding Activity of Double-stapled SAH-MPER Peptides and Structural Analysis of the 10E8 Fab–SAH-MPER(662-683KKK)(B,q) Complex.
(a) Comparative binding activity of double-stapled SAH-MPER(662-683) peptides for 10E8, as measured by competitive ELISA assay against the unmodified 10E8 ligand RRRNEQELLELDKWASLWNWFDITNWLWYIRRRR10. (b) Crystal structure of SAH-MPER(662-683KKK)(B,q) (shown as a cyan ribbon with stick residues) bound to 10E8 Fab at 4.15 Å resolution. (c) Superimposition of the 4E10 Fab–SAH-MPER(671-683KKK)(q) and 10E8 Fab–SAH-MPER(662-683KKK)(B,q) complexes highlights how the two anti-HIV Fabs differentially engage the stapled MPER antigens.