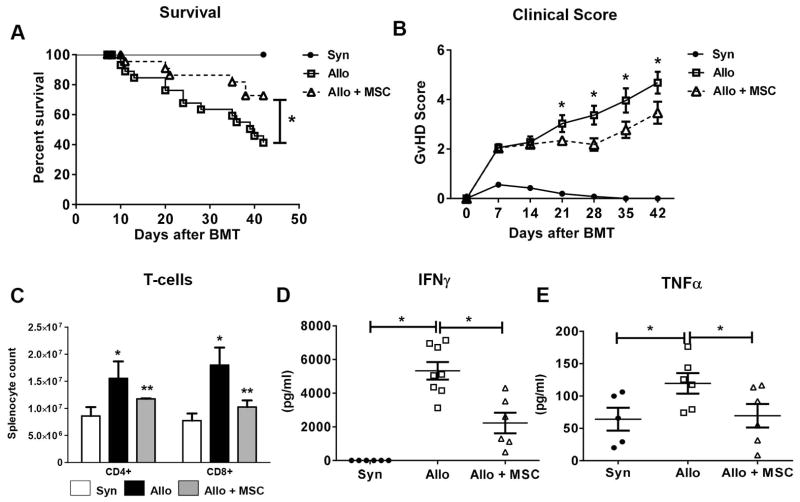
Figure 1. Human MSC infusions (hMSCs) regulate in vivo T-cell expansion, pro-inflammatory cytokine secretion and systemic GvHD following alloBMT.
(A) hMSC infusions reduce systemic GvHD following alloBMT as measured by survival. Data are expressed as mean ± SEM and represent three combined transplant experiments [(n= 15 to 30 mice per experimental group; * p < 0.05 allo control vs. allo + MSC by Mantel-Cox log-rank test (survival) and unpaired t-test (clinical score)]. (B) hMSC infusions improve clinical GvHD scores in alloBMT mice. Combined clinical scores (mean ± SEM) from three separate transplant experiments are depicted. Asterisks (*) indicate significant differences in mean clinical GvHD scores between indicted alloBMT groups at listed post-transplant times (p<0.05, unpaired t-test). (C) hMSC infusions result in decreased donor T-cell expansion at D10. Data are expressed as mean ± SEM and are from one of three representative independent experiments (n=3 to 6 mice per group; * p < 0.05 syn vs. allo control; ** p < 0.05 allo control vs. allo + MSC by Mann-Whitney test). (D, E) AlloBMT mice receiving MSC infusions have decreased levels of circulating IFNγ (D) and TNFα (E). Whole blood was collected, serum separated and ELISA used to measure cytokine levels from individualized mice at D7 (IFNγ) and D10 (TNFα). Data are expressed as mean ± SEM and are from one of three independent experiments (n=5 to 6 mice per experimental group; * p < 0.05 for indicated comparisons by Mann-Whitney test).