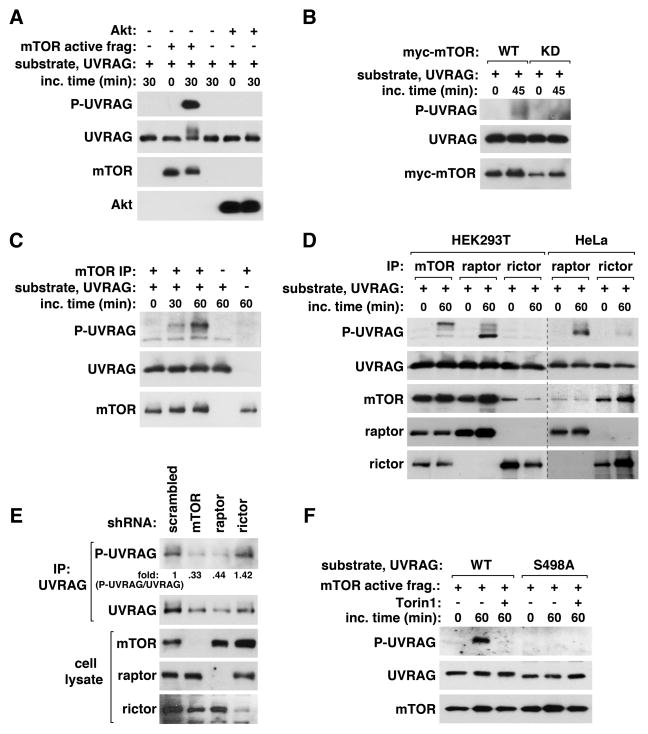
Figure 3. mTORC1 phosphorylates UVRAG Ser498.
(A) mTOR phosphorylates UVRAG S498 in vitro. The active form of mTOR was incubated with puririfed UVRAG in the presence of ATP. Akt1 was used as a negative control. UVRAG S498 phosphorylation was analyzed by WB. (B) Myc-mTOR WT or its kinase dead mutant (KD), transiently expressed in HEK293T cells, was isolated by immunoprecipitation using anti-myc antibody and incubated with UVRAG and ATP. (C) Endogenous mTOR IP was isolated from HEK293T cells, and its kinase activity was analyzed using UVRAG 271–699 fragment as substrate. (D) mTOR, raptor, or rictor IPs were obtained from HEK293T or HeLa cells, and the kinase reaction was analyzed as in (C). (E) The phosphorylation state of S498 was analyzed for endogenous UVRAG isolated from shRNA-transduced HEK293T cells. (F) Torin1 inhibits UVRAG S498 phosphorylation in vitro. The active fragment of mTOR was incubated with UVRAG WT or S498A purified from bacteria. The kinase reaction was performed in the presence or absence of Torin1. See also Figure S3.