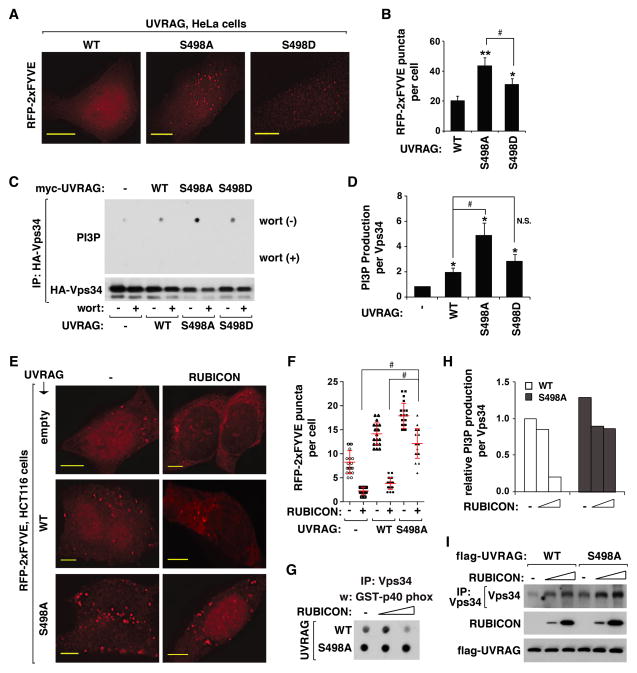
Figure 5. UVRAG Ser498 phosphorylation is important for the inhibitory effect of RUBICON on Vps34.
(A) Prevention of S498 phosphorylation increased the accumulation of PI3P in HeLa cells. RFP-tagged 2xFYVE, transiently expressed in WT or mutant UVRAG-reconstituted HeLa cells, was monitored by fluorescence microscope. Scale bar, 10 μm. (B) Quantitative analysis of FYVE puncta formation. Results are represented as means ± standard deviation (SD) (*, p<0.05 vs WT; **, p<0.01 vs WT; #, p<0.01; n≥17). (C) Prevention of S498 phosphorylation increased the kinase activity of Vps34. HA-Vps34 was expressed alone or together with myc-UVRAG in UVRAG-depleted HEK293T cells. HA-Vps34 IP was obtained and incubated with phosphatidylinositol (PI) and ATP in the presence or absence of wortmannin (200 nM). The amount of PI3P was analyzed as described in Experimental Procedures. (D) Quantitative analysis of Vps34 kinase activity. Results are represented as means ± SD (*, p<0.01 vs empty vector; #, p<0.01; NS, Non-Significant; n=3). (E) RUBICON depends on S498 phosphorylation to suppress UVRAG-induced production of PI3P. RFP-2xFYVE expressed alone (−) or together with RUBICON in empty vector- or UVRAG-transduced HCT116 cells was analyzed as described in (A). Scale bar, 5 μm. (F) Quantitative analysis of PI3P puncta formation. The error bars represent means ± SD (#, p<0.01; n=20). (G and H) RUBICON depends on S498 phosphorylation to suppress UVRAG-mediated stimulation of Vps34 kinase activity. UVRAG-reconstituted HEK293T cells were transiently transfected with empty vector or RUBICON construct. The in vitro kinase assay was conducted as in (C). The graph is representative of two independent experiments. (I) Vps34 recovered with Vps34 IP was analyzed by WB. See also Figure S5.