Abstract
Recently, multivariate random-effects meta-analysis models have received a great deal of attention, despite its greater complexity compared to univariate meta-analyses. One of its advantages is its ability to account for the within-study and between-study correlations. However, the standard inference procedures, such as the maximum likelihood or maximum restricted likelihood inference, require the within-study correlations, which are usually unavailable. In addition, the standard inference procedures suffer from the problem of singular estimated covariance matrix. In this paper, we propose a pseudolikelihood method to overcome the aforementioned problems. The pseudolikelihood method does not require within-study correlations and is not prone to singular covariance matrix problem. In addition, it can properly estimate the covariance between pooled estimates for different outcomes, which enables valid inference on functions of pooled estimates, and can be applied to meta-analysis where some studies have outcomes missing completely at random. Simulation studies show that the pseudolikelihood method provides unbiased estimates for functions of pooled estimates, well-estimated standard errors, and confidence intervals with good coverage probability. Furthermore, the pseudolikelihood method is found to maintain high relative efficiency compared to that of the standard inferences with known within-study correlations. We illustrate the proposed method through three meta-analyses for comparison of prostate cancer treatment, for the association between paraoxonase 1 activities and coronary heart disease, and for the association between homocysteine level and coronary heart disease. © 2014 The Authors. Statistics in Medicine Published by John Wiley & Sons Ltd.
Keywords: composite likelihood, correlation, multivariate meta-analysis, singular estimated covariance matrix problem, pseudolikelihood
1. Introduction
The rapid growth of evidence-based medicine has led to dramatically increasing attention to meta-analysis, which combines statistical evidence from multiple studies. In many randomized clinical trials or observational studies, multiple and possibly correlated outcomes of interest need to be meta-analyzed. Conventionally, univariate methods are used to investigate one outcome at a time, such as pooling the summary measures from all studies through fixed or random effects model 1. These methods gain their popularity because of their usefulness and simplicity. However, if the objective of inference is to compare the overall effects between types of outcomes, such as comparing the risks of a certain disease between two groups, calculating the weighted sums of the estimated sensitivity and specificity in diagnostic tests, or comparing the effect sizes with respect to two endpoints, univariate methods are not sufficient because they cannot account for the possible correlations between multiple outcomes 2–4.
Recently, multivariate meta-analysis (MMA) has received a great deal of attention 5. MMA jointly analyzes multiple and possibly correlated outcomes, which can account for two types of correlations: the within-study correlation and the between-study correlation. The within-study correlation ρWi exists because the effects are sometimes measured using the same set of subjects, such as the overall and disease-free survival of cancer patients. The between-study correlation ρB allows the underlying effects to be correlated. In MMA, a ‘two-stage’ approach for inference is often adopted. At the first stage, analyses of each study are performed, and estimates of effects, referred to as summary measures, are obtained. These estimates are then combined at the second stage by a random effects model. The overall effect sizes or their comparative measures can be inferred using maximum likelihood (ML) or restricted ML (REML) inference based on the marginal distribution of summary measures. For an excellent review of MMA, see Van Houwelingen et al. (2002) 2 and Jackson et al. (2011) 5.
Although conceptually straightforward, conducting MMA faces at least two practical challenges, even when only two outcomes are considered. The first challenge is the lack of knowledge on the within-study correlations ρWi, which are generally required in standard inference procedures for MMA. However, in practice, the within-study correlations are often not reported and are difficult to obtain even on request 6,7. In addition, calculation of the within-study correlation may not be easy and sometimes requires more computationally intensive methods 8. Such challenge is acknowledged by the excellent review paper of Jackson et al., 5, ‘perhaps the greatest difficulty applying the MMA model in practice is that the within-study correlations are required by the model and are typically unknown.’ In such situations, sensitivity analysis with imputed within-study correlations and Bayesian methods have been proposed 9,10. Wei and Higgins 11 recently proposed a practical method for MMA when the within-study correlations are missing. Specifically, they used the information on possible correlations between the underlying outcomes to impute any missing within-study covariances, and then conducted the inference by REML estimation. The second challenge is the (restricted) ML estimate of the between-study correlation ρB is often at or very close to the boundary of its parameter space, that is, ±1. In that case, the estimated covariance matrix is singular. The singular estimated covariance matrix can lead to biased estimates of standard errors and is most severe when the number of studies is small or the within-study variation is relatively large 5,12. As a consequence, the confidence intervals may be too wide or too narrow.
To overcome the aforementioned challenges, Riley et al., 13 proposed a method for bivariate meta-analysis (BMA). Specifically, Riley et al., 13 postulated a synthesis correlation parameter to describe the overall marginal correlation between outcomes. This method is attractive because it does not require within-study correlations and can alleviate the singular estimated covariance matrix problem. However, the prevalence of singular estimated covariance matrix problem can still be substantial in some situations, and the extension from BMA to MMA is not straightforward in order to deal with more than two outcomes. In this paper, we propose an alternative method along the line of the work by Riley et al., 13. The idea is to construct a pseudolikelihood function for overall effect sizes using a working independence assumption. An immediate advantage of the proposed method is the within-study correlations are not required in the construction of pseudolikelihood. The singular estimated covariance matrix problem is resolved because there is no correlation parameter involved in the pseudolikelihood. In fact, the proposed pseudolikelihood belongs to the family of composite likelihoods 14–16. Hence, the pseudolikelihood enjoys the established properties of composite likelihood 14,16–18. In particular, when a working independence assumption is adopted, the pseudolikelihood is called independence likelihood 19, where the covariance between estimates of overall effect sizes can be consistently estimated by the Huber–White standard error estimates, also known as ‘sandwich’ variance estimator 20,21. Another advantage of the pseudolikelihood method is the simplicity of the extension to MMA where more than two outcomes are analyzed, and to missing data situations where some of the multiple outcomes are missing completely at random (MCAR). In this paper, we present the proposed method in the meta-analysis of bivariate outcomes and describe the extension to three or more outcomes that is provided in Section 2.4.
This paper is organized as follows. In Section 2, we describe the standard likelihood inferences for MMA, the method proposed by Riley et al., 13 and the proposed pseudolikelihood method. In Section 3, we extend the proposed method to the missing data situation where only a proportion of studies have all outcomes reported, and so remaining studies have at least one outcome missing. In Section 4, we conduct simulation studies to compare the pseudolikelihood method with the current methods and investigate the prevalence of singular covariance matrix problems, bias, coverage probability (CP), and relative efficiency (RE). We apply the proposed method to three meta-analyses in Section 5. Finally, we provide a brief discussion in Section 6.
2. Statistical methodology
2.1. Bivariate random-effects meta-analysis model
We consider a meta-analysis with m studies where two outcomes in each study are of interest. For the ith study, denote Yij and sij the summary measure for the jth outcome of interest and associated standard error, respectively, both assumed known, i = 1,…,m, and j = 1,2. Each summary measure Yij is an estimate of the true effect size θij. To account for heterogeneity in effect size across studies, we assume θij to be independently drawn from a common distribution with overall effect size βj and between study variance 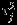 , j = 1,2. Under normal distribution assumption for Yij and θij, the general bivariate random-effects meta-analysis model can be written as 2
, j = 1,2. Under normal distribution assumption for Yij and θij, the general bivariate random-effects meta-analysis model can be written as 2
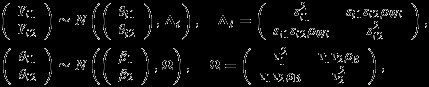 |
1 |
where Δi and Ω are the respective within-study and between-study covariance matrices, and ρWi and ρB are the respective within-study and between-study correlations. When the within-study correlations ρWi are known, inference on the overall effect sizes β1 and β2, or their comparative measures (e.g. β1−β2), can be based on the marginal distribution of (Yi1,Yi2)
We note that the variance of Yij is partitioned into two parts 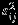 and
and 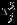 as in analysis of variance for univariate random effects model, and the covariance, cov(Yi1,Yi2) = si1si2ρwi+τ1τ2ρB, is also partitioned into two parts as the sum of within and between study covariances.
as in analysis of variance for univariate random effects model, and the covariance, cov(Yi1,Yi2) = si1si2ρwi+τ1τ2ρB, is also partitioned into two parts as the sum of within and between study covariances.
2.2 Restricted maximum likelihood method
For simplicity of notation, denote Yi=(Yi1,Yi2)T, β = (β1,β2)T, 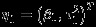 , and
, and 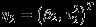 . The restricted likelihood of (η1,η2,ρB) can be written as
. The restricted likelihood of (η1,η2,ρB) can be written as
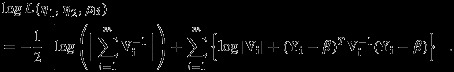 |
The parameters (η1,η2,ρB) can be estimated by the REML approach as described in Van Houwelingen et al., 2. The between-study variances 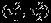 are usually modeled in their log-scale, so that they are forced to be non-negative. REML can be implemented using Newton–Raphson or quasi-Newton methods, as in ‘mvmeta’ package in STATA 22 or R 23. Such methods of implementing REML rarely suffer convergence issues. However, two practical challenges in the standard likelihood inference have been reported 5,13. The first is the lack of knowledge on ρWi. The standard likelihood inference based on the bivariate random-effects meta-analysis requires within-study correlation estimates ρWi, but these are rarely available. The second is the singular estimated covariance matrix problem 13,24. Specifically, a singular covariance matrix is sometimes obtained (i.e., the estimate of the between-study correlation ρB is close to ±1). Consequently, estimates of standard errors are often biased and can lead to confidence intervals that are too conservative or too liberal. As will be illustrated in Section 4, the singular estimated covariance matrix problem can be substantial (e.g., greater than 25% of 1000 samples for meta-analysis with 10 studies) when REML approach is applied. Such a problem is more severe when the ML approach is taken.
are usually modeled in their log-scale, so that they are forced to be non-negative. REML can be implemented using Newton–Raphson or quasi-Newton methods, as in ‘mvmeta’ package in STATA 22 or R 23. Such methods of implementing REML rarely suffer convergence issues. However, two practical challenges in the standard likelihood inference have been reported 5,13. The first is the lack of knowledge on ρWi. The standard likelihood inference based on the bivariate random-effects meta-analysis requires within-study correlation estimates ρWi, but these are rarely available. The second is the singular estimated covariance matrix problem 13,24. Specifically, a singular covariance matrix is sometimes obtained (i.e., the estimate of the between-study correlation ρB is close to ±1). Consequently, estimates of standard errors are often biased and can lead to confidence intervals that are too conservative or too liberal. As will be illustrated in Section 4, the singular estimated covariance matrix problem can be substantial (e.g., greater than 25% of 1000 samples for meta-analysis with 10 studies) when REML approach is applied. Such a problem is more severe when the ML approach is taken.
2.3. Riley method
To account for the aforementioned practical issues of REML method, Riley et al., 13 proposed a new method, which we refer as Riley method hereafter. Specifically, instead of partition, the overall covariance into within and between study covariances, Riley et al. (2008) 13 proposed a synthesis correlation parameter ρS to account for the marginal correlation between Yi1 and Yi2. Following the notations in Riley et al., 13, the new marginal model for (Yi1,Yi2) is
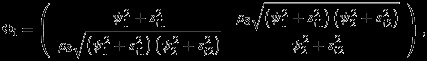 |
4 |
where 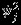 's account for the additional variation beyond the within-study variances
's account for the additional variation beyond the within-study variances 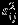 , j = 1,2, and ρS accounts for the marginal correlation between Yi1 and Yi2. Note that
, j = 1,2, and ρS accounts for the marginal correlation between Yi1 and Yi2. Note that 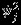 is not equivalent to the between-study variation
is not equivalent to the between-study variation 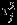 because the model (2) does not have a fully hierarchical structure. Riley et al., 13 proposed to base the inference on the restricted log-likelihood defined as
because the model (2) does not have a fully hierarchical structure. Riley et al., 13 proposed to base the inference on the restricted log-likelihood defined as
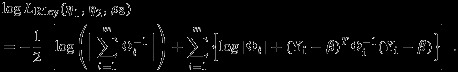 |
The method based on the model (2) has the attractive features of not requiring knowledge on within-study correlations ρWi and being able to account for the correlation between Yi1 and Yi2. In addition, Riley method was found to be less prone to the estimation problem compared to standard likelihood inferences based on REML 13. However, as acknowledged by Riley et al., 13, the problem of unstable estimates when estimate of ρS is close to 1 or −1 is still an issue especially when the between-study heterogeneity is small relative to the within-study variation. In addition, the extension from BMA to MMA with more than two outcomes is not straightforward because multiple synthesis correlation parameters may be required to describe the pairwise marginal correlations between pairs of outcomes. In fact, the ‘mvmeta’ package in STATA by Ian White has recently extended Riley method to multivariate data with more than two outcomes 22. However, it is expected that increasing the number of outcomes is likely to increase the chance of not well-defined correlation estimates because of the need of estimating increased number of correlation parameters.
2.4. Pseudo-restricted maximum likelihood method
Now we propose an alternative method to overcome the aforementioned challenges, which we refer as Pseudo-REML method hereafter. Our strategy is to base the inference of (η1,η2) on a pseudolikelihood function constructed as follows. Under the working independence assumption (i.e., setting ρWi=ρB=0 in the joint distribution of Yi1 and Yi2), we obtain the following pseudolikelihood
| 5 |
where
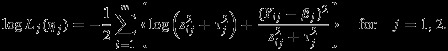 |
6 |
We note that logLj(ηj) is simply the log likelihood for ηj when a univariate random effects model for meta-analysis is assumed. Therefore, the Pseudo-REML method indeed provides the same point and variance estimates as that from the univariate method as far as onlyβ1 (or β2) is concerned. The main difference between the Pseudo-REML method and the univariate method is that the model-based standard error of the difference between β1 and β2 (or any functions of β1 and β2) can be correctly estimated by the Pseudo-REML method, but not by two separate univariate meta-analyses due to the ignored covariance between the estimated β1 and β2. In other words, the Pseudo-REML method can be considered as an inferential strategy for REML or Riley method using working independence assumption.
Note that the score equation for ηj, ∂ logLp(η1,η2)/∂ηj=0 can be calculated as ∂ logLj(ηj)/∂ηj=0, hence is unbiased. The maximum pseudolikelihood estimator  , defined as a solution of the score equation, can be shown to be consistent and asymptotically normal with covariance matrix
, defined as a solution of the score equation, can be shown to be consistent and asymptotically normal with covariance matrix
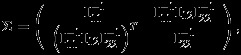 |
where
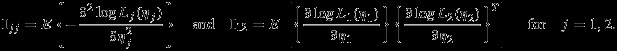 |
The general asymptotic results of composite likelihood have been provided by Kent 17, Lindsay 14, and Molenberghs and Verbeke 18. For the interest of readers, an outline of derivation for the covariance is provided in the Appendix. We note that the pseudolikelihood logLp(η1,η2) is generally not a true likelihood function unless all correlations are truly zero (i.e., setting ρWi=ρB=0 in the joint distribution of Yi1 and Yi2). Consequently, the conventional covariance matrix estimator 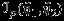 , where
, where 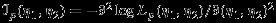 , is no longer valid because
, is no longer valid because 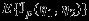 is not the covariance of ∂Lp(η1,η2)/∂(η1,η2) in the presence of correlations.
is not the covariance of ∂Lp(η1,η2)/∂(η1,η2) in the presence of correlations.
The information matrices I11, I22, and I12 can be empirically estimated as
and
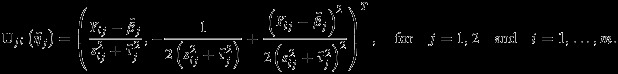 |
The Pseudo-REML method is simple to implement in practice. Computationally, the point estimate of overall effect size 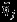 is the same as that from univariate meta-analysis of
is the same as that from univariate meta-analysis of 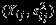 using a random effects model based on REML 1. The covariance matrix of
using a random effects model based on REML 1. The covariance matrix of  can be easily calculated using the aforementioned closed-form formulas. For example, if one was interested in the function β1−β2, then one would take the difference in the univariate pooled estimates for outcomes 1 and 2, and the variance of β1−β2 can then be calculated by
can be easily calculated using the aforementioned closed-form formulas. For example, if one was interested in the function β1−β2, then one would take the difference in the univariate pooled estimates for outcomes 1 and 2, and the variance of β1−β2 can then be calculated by 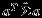 where a = (1,0,−1,0)T.
where a = (1,0,−1,0)T. 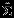 is a 4 × 4 matrix here, generally with dimensions 2K × 2K and K as the number of outcomes. We implement the Pseudo-REML method in R. We use the ‘mvmeta’ function in the R (R Development Core Team, Version 2.14.1) package ‘mvmeta’ to obtain the REML estimates 23. R codes are attached in the Supplemental Materials. We note that the matrix
is a 4 × 4 matrix here, generally with dimensions 2K × 2K and K as the number of outcomes. We implement the Pseudo-REML method in R. We use the ‘mvmeta’ function in the R (R Development Core Team, Version 2.14.1) package ‘mvmeta’ to obtain the REML estimates 23. R codes are attached in the Supplemental Materials. We note that the matrix 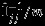 , j = 1,2 is the same as the covariance estimated from the univariate meta-analysis of
, j = 1,2 is the same as the covariance estimated from the univariate meta-analysis of 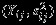 , j = 1,2, whereas the matrix
, j = 1,2, whereas the matrix 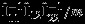 accounts for the covariance between the estimated overall effect sizes
accounts for the covariance between the estimated overall effect sizes 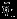 and
and 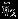 . Properly accounting such covariance is not available if investigators conduct separate univariate meta-analyses on
. Properly accounting such covariance is not available if investigators conduct separate univariate meta-analyses on 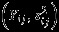 .
.
It is easy to see that the Pseudo-REML method resolves the two practical issues in standard inference of REML method. Specifically, the within-study correlations ρWi are not required in the construction of pseudolikelihood. And the singular estimated covariance matrix problem is resolved because there is no correlation parameter (i.e., ρB or ρS) involved in the pseudolikelihood. Furthermore, the Pseudo-REML method can be easily extended to MMA where more than two outcomes are analyzed. Specifically, for MMA with K outcomes, the corresponding pseudolikelihood becomes 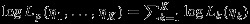 where logLk(ηk) is defined in equation (6). The corresponding covariance matrix can be derived similarly. However, one potential drawback of the Pseudo-REML method when assuming the data are complete or MCAR is that because the point estimates of overall effect sizes are the same as that from separate univariate meta-analyses, there is potential efficiency loss compared to the methods accounting for the correlations between dependent outcomes. We investigate this potential drawback in Section 4.
where logLk(ηk) is defined in equation (6). The corresponding covariance matrix can be derived similarly. However, one potential drawback of the Pseudo-REML method when assuming the data are complete or MCAR is that because the point estimates of overall effect sizes are the same as that from separate univariate meta-analyses, there is potential efficiency loss compared to the methods accounting for the correlations between dependent outcomes. We investigate this potential drawback in Section 4.
3. Extension to missing data where only a subset of outcomes is reported
In Section 2, we assumed that all outcomes of each study are observed. In practice, however, it is common that only a proportion of studies have all outcomes reported, and the remaining studies have some of outcomes missing. In this section, we discuss extension of the Riley method and Pseudo-REML method for missing data situations. In this paper, we only consider the situation that the outcomes are MCAR, which has been considered in 13. Although such assumption often does not hold in practice, it is instructive to consider MCAR setting as a step toward missing at random (MAR) and missing not at random (MNAR) situation. Extension of the Pseudo-REML method to MAR and MNAR is beyond the scope of this paper and is discussed in Section 6.
For simplicity of notations, we assume that two endpoints are of interest. Consider a meta-analysis of m studies where the first m1 studies reported both endpoints, the next m2 studies reported the first endpoint only, and the remaining m3 studies reported the second endpoint only. The restricted log-likelihood for the model proposed by Riley et al., 13 in the missing data situation can be written as
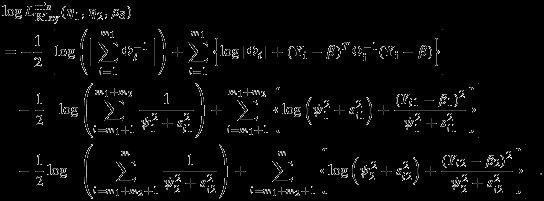 |
The pseudolikelihood in the missing data situation can be defined as
| 12 |
where
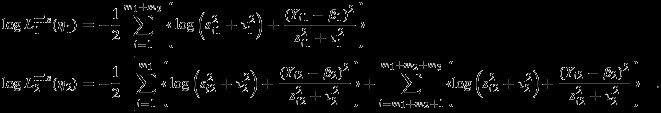 |
We note that when the full data are available, the parameter estimators reduce to those in Section 2.4. The score equation for 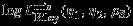 and
and 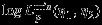 is unbiased if the data are MCAR 25. Assume the cumulative proportions m1/m→r1>0,(m1+m2)/m→r2>0,(m1+m3)/m→r3>0 when m→∞, the maximum pseudolikelihood estimator
is unbiased if the data are MCAR 25. Assume the cumulative proportions m1/m→r1>0,(m1+m2)/m→r2>0,(m1+m3)/m→r3>0 when m→∞, the maximum pseudolikelihood estimator  , can be shown to be consistent and asymptotically normal with covariance matrix
, can be shown to be consistent and asymptotically normal with covariance matrix
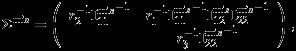 |
where
 |
An outline of derivation for the asymptotic covariance is provided in the Appendix. The covariance matrix Σmis can be empirically estimated as detailed in Appendix.
4. Simulation study
4.1. Methods under comparison
In this section, we evaluate the finite sample performance of the Pseudo-REML method and compare it to that of REML and Riley methods. The data are generated from a two-stage procedure as specified by equation (1). To cover a wide spectrum of scenarios, we vary the values for four factors that are considered important in practice. Specifically, the number of studies is set to 10 or 25 to represent meta-analysis of a moderate number or large number of studies, respectively. We consider both the complete data and missing data scenarios. For missing data scenario, we assume 40% of studies have one of two outcome MCAR. To reflect the heterogeneity in standard error of summary measure across studies, we sample 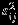 from the square of N(0.25,0.50) distribution, which leads to a median value of 0.25 for
from the square of N(0.25,0.50) distribution, which leads to a median value of 0.25 for 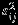 . The size of the within-study variation relative to the between-study variation may have a substantial impact on the performance of the methods. To this end, we let the ratio of the within-study variation relative to the between study variation to be relatively small, comparable, and large, corresponding to
. The size of the within-study variation relative to the between-study variation may have a substantial impact on the performance of the methods. To this end, we let the ratio of the within-study variation relative to the between study variation to be relatively small, comparable, and large, corresponding to 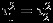 =0.5, 0.25, or 0.1, respectively. For within-study correlations, we consider two different settings: one is ρWi being constant with value −0.5, 0, or 0.5, the other is ρWi varying across studies and being randomly sampled from Uniform (−0.8,0.8), Uniform (−0.8,0) and Uniform (0,0.8) to reflect arbitrary, negative, and positive within-study correlations, respectively. The between-study correlation ρB is set to −0.5, 0, or 0.5. We set the overall effect sizes to be β1=β2=0 and denote the difference in effect size between two outcomes by δ = β1−β2. For each simulation setting, we generate 5000 samples. The samples are simulated in R using the ‘mvrnorm’ function. Each of the simulated data is analyzed by three methods, namely, REML, Riley method, and the Pseudo-REML method. We note that REML method, although not applicable in practice due to the unknown within-study correlations, can serve as a gold standard for comparison. The results from REML method are obtained from the ‘mvmeta’ function in the R package ‘mvmeta’.
=0.5, 0.25, or 0.1, respectively. For within-study correlations, we consider two different settings: one is ρWi being constant with value −0.5, 0, or 0.5, the other is ρWi varying across studies and being randomly sampled from Uniform (−0.8,0.8), Uniform (−0.8,0) and Uniform (0,0.8) to reflect arbitrary, negative, and positive within-study correlations, respectively. The between-study correlation ρB is set to −0.5, 0, or 0.5. We set the overall effect sizes to be β1=β2=0 and denote the difference in effect size between two outcomes by δ = β1−β2. For each simulation setting, we generate 5000 samples. The samples are simulated in R using the ‘mvrnorm’ function. Each of the simulated data is analyzed by three methods, namely, REML, Riley method, and the Pseudo-REML method. We note that REML method, although not applicable in practice due to the unknown within-study correlations, can serve as a gold standard for comparison. The results from REML method are obtained from the ‘mvmeta’ function in the R package ‘mvmeta’.
4.2. Simulation results
Table 1 summarizes empirical bias (Bias), empirical standard error (ESE), model-based standard error estimates, and the CP of the confidence intervals for δ when the within-study/between-study variation ratio is close to 1 (i.e., median of 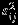 is 0.25 and
is 0.25 and 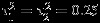 ) for complete and missing data settings. The ESE is calculated as the standard deviation of point estimates, and model-based standard error estimate is calculated as the average of model-based standard errors. As shown in the upper panel of Table 1, for the complete data settings, all three methods provide unbiased estimates of δ. When the number of studies is moderate (m = 10), Riley method tends to underestimate the standard error, leading to confidence intervals with coverage probabilities that are less than the nominal level (range of CP: 89.1 ∼ 92.0%). The possible reason is that Riley method performs well only when estimated
) for complete and missing data settings. The ESE is calculated as the standard deviation of point estimates, and model-based standard error estimate is calculated as the average of model-based standard errors. As shown in the upper panel of Table 1, for the complete data settings, all three methods provide unbiased estimates of δ. When the number of studies is moderate (m = 10), Riley method tends to underestimate the standard error, leading to confidence intervals with coverage probabilities that are less than the nominal level (range of CP: 89.1 ∼ 92.0%). The possible reason is that Riley method performs well only when estimated 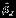 is relatively small. In contrast, the standard error of estimate for δ using the Pseudo-REML method is well estimated, and the coverage probabilities of confidence intervals are close to the nominal level (range of CP: 94.9 ∼ 95.8%). To assess the singular covariance matrix problem, we display the percentage of the singular covariance matrix (SP) under different settings when m = 10 in Figure S2 of the Supplemental Materials. We find that the REML method suffers greatly from the singular estimated covariance matrix problem (SP>25% under all settings). Riley method alleviates this problem to some extent but still has a sizable proportion of singular estimated covariance matrix in some settings (range of SP: 0 ∼ 20%). In contrast, there is no singular estimated covariance matrix problem for the Pseudo-REML method. We further examine the performance of estimates under three different methods when the estimated covariance matrix using REML method is singular. When REML method has singular estimated covariance matrix problem, the point estimates from three methods are similar, but their standard error estimates are quite different due to the singularity of estimated covariance matrix, in that the standard errors are larger in the REML method. This was also noted by Riley et al. (2007) 12. The details can be found in Figure S3 of the Supplemental Materials. When the number of studies is relatively large (m = 25), both Riley method and the Pseudo-REML method perform well in that estimates are unbiased and confidence intervals have coverage probabilities close to the nominal level.
is relatively small. In contrast, the standard error of estimate for δ using the Pseudo-REML method is well estimated, and the coverage probabilities of confidence intervals are close to the nominal level (range of CP: 94.9 ∼ 95.8%). To assess the singular covariance matrix problem, we display the percentage of the singular covariance matrix (SP) under different settings when m = 10 in Figure S2 of the Supplemental Materials. We find that the REML method suffers greatly from the singular estimated covariance matrix problem (SP>25% under all settings). Riley method alleviates this problem to some extent but still has a sizable proportion of singular estimated covariance matrix in some settings (range of SP: 0 ∼ 20%). In contrast, there is no singular estimated covariance matrix problem for the Pseudo-REML method. We further examine the performance of estimates under three different methods when the estimated covariance matrix using REML method is singular. When REML method has singular estimated covariance matrix problem, the point estimates from three methods are similar, but their standard error estimates are quite different due to the singularity of estimated covariance matrix, in that the standard errors are larger in the REML method. This was also noted by Riley et al. (2007) 12. The details can be found in Figure S3 of the Supplemental Materials. When the number of studies is relatively large (m = 25), both Riley method and the Pseudo-REML method perform well in that estimates are unbiased and confidence intervals have coverage probabilities close to the nominal level.
Table I.
Estimates of bias, empirical standard error (ESE), model-based standard error (MBSE), coverage probability (CP) of δ = β1−β2 in 5000 simulations based on data generated from restricted maximum likelihood (REML) model, with the within-study/between-study variation ratio close to 1, for different number of studies m and different between-study correlation ρB and within-study correlations ρWi. All entries in the table are multiplied by 100.
| REML |
Riley method |
Pseudo-REML |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m | Scenarios | ρWi | ρB | Bias | ESE | MBSE | CP | Bias | ESE | MBSE | CP | Bias | ESE | MBSE | CP | |||
| 10 | Complete | −0.5 | −0.5 | −0.7 | 38.0 | 33.5 | 87.5 | −0.6 | 38.8 | 35.8 | 89.9 | −0.8 | 37.8 | 43.4 | 95.2 | |||
| 0.0 | 0.6 | 35.3 | 30.6 | 86.5 | 0.5 | 35.2 | 33.6 | 91.0 | 0.5 | 34.5 | 40.3 | 95.2 | ||||||
| 0.5 | 0.2 | 30.7 | 26.6 | 87.6 | 0.3 | 30.9 | 30.2 | 92.0 | 0.3 | 30.2 | 36.9 | 95.8 | ||||||
| 0 | −0.5 | −0.5 | 35.9 | 32.4 | 88.5 | −0.4 | 36.7 | 33.7 | 90.0 | −0.6 | 35.8 | 41.1 | 95.0 | |||||
| 0.0 | 0.4 | 33.1 | 29.0 | 87.5 | 0.4 | 32.9 | 31.0 | 90.9 | 0.5 | 32.3 | 38.0 | 95.0 | ||||||
| 0.5 | 0.3 | 28.2 | 24.6 | 88.2 | 0.4 | 28.1 | 27.1 | 91.6 | 0.4 | 28.0 | 34.5 | 95.7 | ||||||
| 0.5 | −0.5 | −0.4 | 33.4 | 30.5 | 88.9 | −0.4 | 34.6 | 31.4 | 89.1 | −0.5 | 34.0 | 38.7 | 94.9 | |||||
| 0.0 | 0.4 | 29.8 | 26.8 | 88.4 | 0.4 | 30.2 | 28.1 | 90.7 | 0.5 | 29.9 | 35.7 | 95.3 | ||||||
| 0.5 | 0.4 | 25.1 | 21.9 | 87.5 | 0.5 | 25.0 | 23.5 | 90.7 | 0.4 | 25.6 | 31.6 | 95.4 | ||||||
| Missing | −0.5 | −0.5 | −0.3 | 43.1 | 36.9 | 86.0 | 0.1 | 45.9 | 39.3 | 86.2 | -0.4 | 43.2 | 53.9 | 95.7 | ||||
| 0.0 | 0.4 | 42.2 | 35.9 | 86.5 | 0.5 | 44.8 | 39.0 | 87.2 | 0.5 | 41.4 | 52.2 | 95.9 | ||||||
| 0.5 | 0.1 | 39.2 | 33.9 | 87.9 | -0.1 | 41.0 | 37.1 | 88.9 | 0.0 | 37.9 | 50.1 | 96.3 | ||||||
| 0 | −0.5 | 0.1 | 42.4 | 36.0 | 85.9 | 0.0 | 44.3 | 38.7 | 87.6 | -0.1 | 41.7 | 52.5 | 96.0 | |||||
| 0.0 | 0.4 | 41.0 | 34.4 | 85.3 | 0.6 | 42.9 | 37.9 | 88.0 | 0.5 | 39.9 | 50.7 | 95.8 | ||||||
| 0.5 | 0.3 | 37.2 | 31.7 | 87.2 | 0.0 | 38.8 | 35.4 | 89.1 | 0.1 | 36.3 | 48.6 | 96.6 | ||||||
| 0.5 | −0.5 | −0.1 | 41.1 | 34.5 | 85.2 | 0.0 | 42.6 | 37.5 | 88.1 | −0.1 | 40.3 | 50.9 | 96.1 | |||||
| 0.0 | 0.7 | 38.9 | 31.9 | 85.1 | 0.5 | 40.5 | 36.0 | 88.4 | 0.7 | 38.2 | 49.2 | 95.8 | ||||||
| 0.5 | 0.4 | 34.8 | 28.5 | 85.2 | 0.1 | 36.1 | 33.1 | 89.3 | 0.0 | 34.8 | 46.5 | 96.6 | ||||||
| 25 | Complete | −0.5 | −0.5 | 0.0 | 23.3 | 22.1 | 92.1 | −0.1 | 23.3 | 22.5 | 92.9 | 0.1 | 23.5 | 24.8 | 95.0 | |||
| 0.0 | −0.1 | 21.0 | 19.9 | 91.4 | −0.1 | 21.0 | 21.2 | 94.0 | −0.1 | 20.9 | 22.6 | 95.2 | ||||||
| 0.5 | 0.3 | 18.8 | 16.7 | 89.3 | 0.1 | 18.9 | 18.9 | 94.1 | 0.1 | 18.9 | 20.1 | 95.5 | ||||||
| 0 | −0.5 | −0.1 | 22.2 | 21.3 | 92.3 | −0.1 | 22.2 | 21.2 | 92.6 | −0.1 | 22.2 | 23.5 | 95.0 | |||||
| 0.0 | −0.1 | 19.9 | 18.9 | 91.8 | 0.0 | 19.7 | 19.4 | 93.4 | 0.0 | 19.6 | 21.2 | 95.0 | ||||||
| 0.5 | 0.2 | 17.3 | 15.6 | 90.1 | 0.1 | 17.1 | 17.0 | 94.0 | 0.1 | 17.2 | 18.6 | 95.8 | ||||||
| 0.5 | −0.5 | −0.2 | 20.5 | 20.0 | 92.7 | −0.2 | 20.9 | 19.8 | 92.2 | −0.2 | 20.8 | 22.2 | 94.8 | |||||
| 0.0 | 0.0 | 18.2 | 17.5 | 92.3 | 0.0 | 18.2 | 17.5 | 92.6 | 0.0 | 18.3 | 19.7 | 94.6 | ||||||
| 0.5 | 0.2 | 15.2 | 14.2 | 91.4 | 0.1 | 15.2 | 14.7 | 93.7 | 0.0 | 15.5 | 17.0 | 95.7 | ||||||
| Missing | −0.5 | −0.5 | −0.2 | 26.4 | 24.3 | 90.7 | −0.1 | 26.6 | 25.0 | 91.7 | −0.1 | 26.6 | 29.0 | 95.1 | ||||
| 0.0 | −0.3 | 25.5 | 23.0 | 90.2 | −0.1 | 25.5 | 24.5 | 92.7 | −0.2 | 24.8 | 27.7 | 95.6 | ||||||
| 0.5 | 0.0 | 24.2 | 21.1 | 88.8 | −0.2 | 24.1 | 23.4 | 93.4 | −0.1 | 23.6 | 26.2 | 95.9 | ||||||
| 0 | −0.5 | −0.4 | 26.0 | 23.8 | 90.6 | −0.3 | 26.0 | 24.6 | 91.7 | −0.3 | 25.8 | 28.1 | 95.1 | |||||
| 0.0 | −0.2 | 24.6 | 22.2 | 89.6 | −0.1 | 24.4 | 23.7 | 93.0 | −0.1 | 23.9 | 26.8 | 95.8 | ||||||
| 0.5 | −0.1 | 22.9 | 20.1 | 89.1 | −0.2 | 22.5 | 22.2 | 93.9 | −0.2 | 22.4 | 25.3 | 96.4 | ||||||
| 0.5 | −0.5 | −0.5 | 25.3 | 23.0 | 90.3 | −0.4 | 25.3 | 23.8 | 91.7 | −0.5 | 25.0 | 27.3 | 94.9 | |||||
| 0.0 | −0.1 | 23.3 | 21.2 | 89.6 | −0.1 | 23.2 | 22.5 | 92.3 | −0.1 | 23.1 | 25.9 | 95.5 | ||||||
| 0.5 | −0.2 | 21.3 | 18.5 | 88.4 | −0.2 | 20.9 | 20.8 | 94.4 | −0.3 | 21.3 | 24.4 | 96.6 | ||||||
Table 2 summarizes the similar results as in Table 1 when the within-study/between-study variation ratio is close to 2.5 (i.e., median of 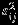 is 0.25 and
is 0.25 and 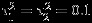 ). For the complete data settings when the number of studies is moderate (m = 10), the ranges of CPs are 86.4 ∼ 88.7% for REML method, 88.4 ∼ 91.6% for Riley method, and 95.5 ∼ 96.6% for the Pseudo-REML method, respectively. When the number of studies is relatively large (m = 25), both Riley method and the Pseudo-REML method perform well. We find that when the between-study heterogeneity is relatively small (i.e., the within-study/between-study variation ratio is relatively large), there is a substantial increase in the percentage of singular estimated covariance matrix problem in both REML and Riley methods.
). For the complete data settings when the number of studies is moderate (m = 10), the ranges of CPs are 86.4 ∼ 88.7% for REML method, 88.4 ∼ 91.6% for Riley method, and 95.5 ∼ 96.6% for the Pseudo-REML method, respectively. When the number of studies is relatively large (m = 25), both Riley method and the Pseudo-REML method perform well. We find that when the between-study heterogeneity is relatively small (i.e., the within-study/between-study variation ratio is relatively large), there is a substantial increase in the percentage of singular estimated covariance matrix problem in both REML and Riley methods.
Table II.
Estimates of bias, empirical standard error (ESE), model-based standard error (MBSE), coverage probability (CP) of δ = β1−β2 in 5000 simulations based on data generated from restricted maximum likelihood (REML) model, with the within-study/between-study variation ratio close to 2.5, for different number of studies m and different between-study correlation ρB and within-study correlations ρWi. All entries in the table are multiplied by 100.
| REML |
Riley method |
Pseudo-REML |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m | Scenarios | ρWi | ρB | Bias | ESE | MBSE | CP | Bias | ESE | MBSE | CP | Bias | ESE | MBSE | CP | |||
| 10 | Complete | −0.5 | −0.5 | −0.5 | 28.2 | 24.3 | 86.4 | −0.2 | 29.2 | 26.7 | 89.0 | −0.6 | 28.2 | 34.1 | 96.1 | |||
| 0.0 | 0.3 | 27.1 | 23.2 | 86.4 | 0.4 | 27.8 | 26.2 | 91.0 | 0.3 | 26.4 | 32.5 | 95.9 | ||||||
| 0.5 | 0.2 | 24.7 | 21.7 | 88.3 | 0.3 | 25.5 | 25.0 | 91.6 | 0.1 | 24.2 | 30.9 | 96.6 | ||||||
| 0.0 | −0.5 | −0.3 | 26.5 | 23.3 | 87.5 | 0.0 | 27.2 | 25.0 | 89.8 | −0.4 | 26.3 | 31.8 | 96.0 | |||||
| 0.0 | 0.1 | 25.0 | 21.8 | 87.4 | 0.1 | 25.4 | 23.8 | 90.8 | 0.2 | 24.5 | 30.2 | 95.9 | ||||||
| 0.5 | 0.2 | 22.5 | 19.8 | 88.7 | 0.3 | 22.8 | 22.0 | 91.3 | 0.2 | 22.2 | 28.6 | 96.1 | ||||||
| 0.5 | −0.5 | -0.2 | 24.0 | 21.4 | 87.8 | 0.0 | 25.0 | 22.4 | 88.4 | −0.2 | 24.5 | 29.4 | 95.5 | |||||
| 0.0 | 0.1 | 22.0 | 19.4 | 87.9 | 0.1 | 22.4 | 20.7 | 90.2 | 0.2 | 22.4 | 27.8 | 95.7 | ||||||
| 0.5 | 0.2 | 19.4 | 17.0 | 87.8 | 0.3 | 19.4 | 18.3 | 89.8 | 0.2 | 20.0 | 25.8 | 96.1 | ||||||
| Missing | −0.5 | −0.5 | −0.2 | 32.6 | 28.0 | 86.6 | 0.5 | 35.6 | 30.5 | 86.4 | −0.3 | 33.0 | 44.4 | 96.9 | ||||
| 0.0 | 0.1 | 32.2 | 27.9 | 87.1 | 0.2 | 35.2 | 30.6 | 87.3 | 0.1 | 31.8 | 43.6 | 97.2 | ||||||
| 0.5 | 0.0 | 30.8 | 27.4 | 88.4 | −0.1 | 33.2 | 29.9 | 87.9 | −0.1 | 30.1 | 43.1 | 97.6 | ||||||
| 0.0 | −0.5 | 0.1 | 31.8 | 27.4 | 86.5 | −0.1 | 34.3 | 30.0 | 87.5 | −0.1 | 31.5 | 43.1 | 97.0 | |||||
| 0.0 | 0.1 | 31.1 | 26.8 | 86.6 | 0.2 | 33.2 | 29.6 | 88.0 | 0.2 | 30.5 | 42.0 | 97.1 | ||||||
| 0.5 | 0.1 | 29.0 | 25.7 | 88.2 | 0.1 | 31.3 | 28.3 | 88.9 | 0.0 | 28.5 | 41.4 | 97.7 | ||||||
| −0.5 | 0.2 | 30.2 | 25.7 | 86.1 | 0.0 | 32.1 | 28.5 | 87.7 | 0.1 | 29.9 | 41.2 | 97.2 | ||||||
| 0.5 | 0.0 | 0.2 | 29.0 | 24.5 | 85.7 | 0.4 | 31.0 | 27.8 | 87.9 | 0.3 | 29.0 | 40.8 | 97.1 | |||||
| 0.5 | 0.2 | 26.8 | 22.8 | 86.5 | 0.0 | 28.6 | 26.1 | 88.6 | −0.1 | 27.1 | 39.3 | 97.6 | ||||||
| 25 | Complete | −0.5 | −0.5 | −0.1 | 17.1 | 15.6 | 90.1 | 0.0 | 17.3 | 16.3 | 91.8 | 0.1 | 17.3 | 18.2 | 94.6 | |||
| 0.0 | −0.1 | 16.0 | 14.5 | 89.9 | −0.2 | 16.0 | 15.9 | 93.6 | −0.2 | 15.8 | 17.2 | 95.3 | ||||||
| 0.5 | 0.2 | 14.8 | 13.0 | 89.2 | 0.0 | 15.0 | 15.1 | 94.1 | 0.1 | 14.9 | 15.9 | 95.1 | ||||||
| 0.0 | −0.5 | −0.1 | 16.1 | 15.0 | 91.0 | −0.1 | 16.2 | 15.2 | 91.6 | −0.1 | 16.1 | 17.1 | 94.5 | |||||
| 0.0 | −0.1 | 15.0 | 13.6 | 89.8 | −0.1 | 14.8 | 14.4 | 92.6 | −0.1 | 14.8 | 15.9 | 94.9 | ||||||
| 0.5 | 0.1 | 13.6 | 12.0 | 89.3 | 0.0 | 13.4 | 13.3 | 93.8 | 0.0 | 13.5 | 14.6 | 95.8 | ||||||
| 0.5 | −0.5 | −0.2 | 14.5 | 13.8 | 91.8 | −0.1 | 14.9 | 13.8 | 91.0 | −0.2 | 14.8 | 15.9 | 94.7 | |||||
| 0.0 | 0.0 | 13.3 | 12.4 | 90.7 | 0.0 | 13.4 | 12.6 | 91.8 | 0.0 | 13.6 | 14.6 | 94.6 | ||||||
| 0.5 | 0.1 | 11.7 | 10.6 | 90.4 | 0.0 | 11.6 | 11.2 | 93.3 | 0.0 | 12.1 | 13.2 | 95.4 | ||||||
| Missing | −0.5 | −0.5 | −0.1 | 19.7 | 17.6 | 89.8 | −0.1 | 19.9 | 18.5 | 90.7 | 0.0 | 19.8 | 21.8 | 95.1 | ||||
| 0.0 | −0.2 | 19.3 | 17.0 | 88.8 | −0.1 | 19.4 | 18.3 | 91.5 | −0.2 | 18.9 | 21.1 | 95.1 | ||||||
| 0.5 | 0.0 | 18.6 | 16.4 | 89.6 | −0.1 | 18.9 | 18.0 | 92.3 | −0.1 | 18.3 | 20.4 | 95.8 | ||||||
| 0.0 | −0.5 | −0.2 | 19.2 | 17.2 | 90.1 | −0.2 | 19.3 | 18.1 | 91.5 | −0.2 | 19.1 | 21.1 | 95.5 | |||||
| 0.0 | −0.2 | 18.5 | 16.4 | 89.3 | −0.1 | 18.5 | 17.6 | 91.9 | −0.1 | 18.2 | 20.4 | 95.4 | ||||||
| 0.5 | −0.1 | 17.6 | 15.6 | 89.6 | −0.1 | 17.6 | 17.0 | 93.1 | −0.2 | 17.4 | 19.7 | 96.1 | ||||||
| −0.5 | −0.4 | 18.4 | 16.4 | 89.6 | −0.3 | 18.6 | 17.3 | 91.3 | −0.4 | 18.4 | 20.3 | 95.4 | ||||||
| 0.5 | 0.0 | −0.1 | 17.3 | 15.3 | 88.6 | −0.1 | 17.4 | 16.6 | 92.0 | −0.1 | 17.4 | 19.5 | 95.4 | |||||
| 0.5 | −0.2 | 16.1 | 14.1 | 89.1 | −0.2 | 16.1 | 15.8 | 93.1 | −0.2 | 16.5 | 18.8 | 96.5 | ||||||
For missing data settings, there is more advantage in avoiding the singular estimated covariance matrix problem for the Pseudo-REML method when the number of studies is moderate. For example, as shown in the lower panel of Table 2 when m = 10, the ranges of CPs are 85.1 ∼ 87.9% for REML method, 86.2 ∼ 89.3% for Riley method, and 95.7 ∼ 96.6% for the Pseudo-REML method, respectively.
We also consider the settings when the number of studies is relatively small (m = 5) and settings when the within-study/between-study variation ratio is close to 0.5 (i.e., median of 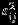 is 0.25 and
is 0.25 and 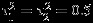 ). The findings are similar to the setting with moderate sample size (m = 10) and hence are not presented here for limited space (see Table S1 in Supplemental Material). In summary, for meta-analysis with moderate number of studies, REML method suffers greatly from the singular estimated covariance matrix problem, even when within-study correlations are known. Riley method alleviates such problem to some extent but still has a sizable proportion of singular estimated covariance matrix. The Pseudo-REML method does not suffer from the singular estimated covariance matrix problem and can produce confidence intervals with coverage probabilities close to the nominal level under all settings considered.
). The findings are similar to the setting with moderate sample size (m = 10) and hence are not presented here for limited space (see Table S1 in Supplemental Material). In summary, for meta-analysis with moderate number of studies, REML method suffers greatly from the singular estimated covariance matrix problem, even when within-study correlations are known. Riley method alleviates such problem to some extent but still has a sizable proportion of singular estimated covariance matrix. The Pseudo-REML method does not suffer from the singular estimated covariance matrix problem and can produce confidence intervals with coverage probabilities close to the nominal level under all settings considered.
One interesting phenomenon shown in Tables2 and 4 is that the ESE from REML method is very close to that from the Pseudo-REML method for the complete data settings when number of studies is moderate or large. This suggests that the efficiency gain in the individual pooled estimates through the joint analysis of Yi1 and Yi2 may not be large for complete data, even when the within-study correlations ρWi are available. Such finding is consistent with the literature; for example, see 26, 24, and 27. To compare the efficiency in the estimation of δ, we consider the RE defined by the square of the ESE of the estimates from REML method, divided by that of Riley method or the Pseudo-REML method. We plot the RE against the between-study correlation ρB in Figure 1 (for within-study/between-study variation ratio close to 1). To cover a wide range of between-study correlations and to include the rare situations of extreme between-study correlations, we let ρB vary from −0.99 to 0.99. As shown in Figure 1, in complete data settings when m = 10, the relative efficiencies of the both Riley method and Pseudo-REML method compared to REML method are close to 100% except the extreme situation where ρB is greater than 0.8.
Table IV.
Results of Riley method and the Pseudo restricted maximum likelihood (Pseudo-REML) method applied to the three meta-analyses.
| Complete data scenario |
Missing data scenario |
|||||||
|---|---|---|---|---|---|---|---|---|
| Study | Method | Pooled estimates | 95% CI | p-value | Pooled estimates | 95% CI | p-value | |
| Sasse † | Riley method | 0.312 | (0.104, 0.519) | 0.003 | — | — | — | |
| Pseudo-REML | 0.320 | (0.054, 0.585) | 0.018 | — | — | — | ||
| Zhao 2012‡ | Riley method | −0.078 | (−0.387, 0.232) | 0.623 | −0.281 | (−0.688, 0.126) | 0.176 | |
| Pseudo-REML | −0.075 | (−0.377, 0.228) | 0.627 | −0.300 | (−0.928, 0.328) | 0.349 | ||
| Thompson 2005* | Riley method | 0.055 | (−0.050, 0.160) | 0.304 | 0.072 | (0.024, 0.121) | 0.004 | |
| Pseudo-REML | 0.076 | (−0.030, 0.181) | 0.159 | 0.070 | (0.030, 0.110) | 0.001 | ||
The pooled estimate is the difference between the log-hazard ratio with respect to the overall survival and disease-free survival.
The pooled estimate is the difference between the standard mean difference comparing cases and controls of paraoxonase activity and that of arylesterase activity.
The pooled estimate is logORTT vs CC/δP, which is the effect of one unit change in homocysteine level on logORTT vs CC.
Figure 1.
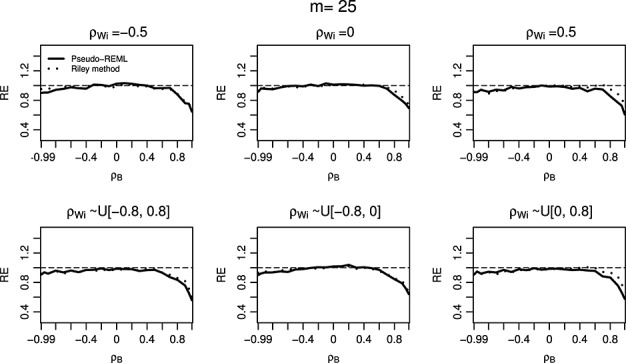
Relative efficiency of estimator of δ = β1−β2 based on Riley method and Pseudo-restricted maximum likelihood (REML) method comparing to the estimator based on REML model, with the within-study/between-study variation ratio close to 1 for different between-study correlation ρB and within-study correlations ρWi. The number of studies m = 10 and number of simulations is 5000.
We evaluated the Type I error and power of REML, Riley method, and Pseudo-REML method for the complete data settings. Table 3 summarizes the Type I error at 5% significance level when the within-study/between-study variation ratio is close to 1. We can see that Pseudo-REML method controls the Type I errors well at all settings considered, whereas the REML and Riley methods have inflated type I errors. It is worth mentioning that if only assessing on results with no singularity problem, the results from the REML method improve substantially in that the type I errors are close to the nominal level. Figure 2 displays the corresponding powers of these three methods when between study correlation ρB=0.5. The critical region that is used to calculate the power is adjusted so that the corresponding Type I error is 5%. The powers of three methods are similar with Pseudo-REML method being slightly more powerful than the other two.
Table III.
Type I error (in %) for restricted maximum likelihood (REML), Riley method, and Pseudo-REML considered at 5% significance level when the within-study/between-study variation ratio is close to 1.
| m | ρWi | ρB | REML | Riley method | Pseudo-REML | |
|---|---|---|---|---|---|---|
| 10 | −0.5 | −0.5 | 12.5 | 10.1 | 4.8 | |
| 0.0 | 13.5 | 9.0 | 4.8 | |||
| 0.5 | 12.4 | 8.0 | 4.2 | |||
| 0.0 | −0.5 | 11.5 | 10.0 | 5.0 | ||
| 0.0 | 12.5 | 9.1 | 5.0 | |||
| 0.5 | 11.8 | 8.4 | 4.3 | |||
| 0.5 | −0.5 | 11.1 | 10.9 | 5.1 | ||
| 0.0 | 11.6 | 9.3 | 4.7 | |||
| 0.5 | 12.5 | 9.3 | 4.6 | |||
| 25 | −0.5 | −0.5 | 7.9 | 7.1 | 5.0 | |
| 0.0 | 8.6 | 6.0 | 4.8 | |||
| 0.5 | 10.7 | 5.9 | 4.5 | |||
| 0.0 | −0.5 | 7.7 | 7.4 | 5.0 | ||
| 0.0 | 8.2 | 6.6 | 5.0 | |||
| 0.5 | 9.9 | 6.0 | 4.2 | |||
| 0.5 | −0.5 | 7.3 | 7.8 | 5.2 | ||
| 0.0 | 7.7 | 7.4 | 5.4 | |||
| 0.5 | 8.6 | 6.3 | 4.3 |
Figure 2.
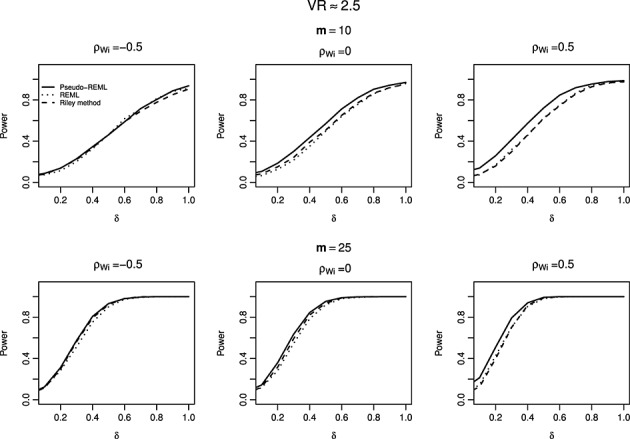
The power curves of restricted maximum likelihood (REML) method, Riley method, and Pseudo-REML method, with the within-study/between-study variation ratio close to 1, for different number of studies m and within-study correlations ρWi. The between-study correlation ρB is 0.5. The number of simulations is 5000.
In summary, our simulation studies suggest that both Riley method and the Pseudo-REML method perform well when the number of studies is large and the Pseudo-REML method does not suffer from the singular estimated covariance matrix problem, and maintains good CP of confidence intervals and high RE when number of studies is relatively small or moderate. Pseudo-REML method has well-controlled Type I error and competitive power compared to Riley method.
5. Applications
We illustrate the Pseudo-REML method by three meta-analyses. For these three meta-analyses, the within-study correlations are unavailable, which is common in practice. As a result, REML method cannot be applied. Alternatively, Riley method and the Pseudo-REML method can be used. In the first example, we consider a meta-analysis of small number of studies where both outcomes are reported, while in the second and third examples, we consider meta-analyses of large number of studies where some studies only have one of two outcomes reported.
5.1. Comparison between overall survival and disease-free survival for prostate cancer
Prostate cancer is a malignant tumor that develops in the prostate and is the sixth leading cause of cancer-related deaths in men 28. Radiotherapy is considered as the most commonly used treatment for locally advanced prostate cancer because of its high survival rate and low morbidity. Androgen deprivation therapy, also called hormone therapy, is a strategy adjuvant to radiotherapy. Recent randomized trials show that androgen deprivation therapy has inhibitory effect on the growth and proliferation of prostate cancer cell. Goserelin acetate, an injectable gonadotropin releasing hormone superagonist, is often used to suppress the production of the sex hormones in the treatment of prostate cancer. Sasse et al., 29 compared the hormone therapy using Goserelin acetate combined with radiotherapy (referred to as ‘combined therapy’ hereafter) versus radiotherapy alone in overall survival and disease-free survival. Five randomized clinical trials published between 1988 and 2011 have reported log-hazard ratio estimates comparing combined therapy using Goserelin acetate with radiotherapy with respect to both overall survival and that with respect to disease-free survival, denoted by Yi1 and Yi2, respectively. It is of clinically importance to evaluate the difference between the log-hazard ratio with respect to overall survival and disease-free survival 30. Denote this difference by δ, we conduct a meta-analysis of the five trials by applying both Riley method and the Pseudo-REML method. As shown in the upper left panel of Table 4, the difference δ is estimated as 0.320 (95% CI: (0.054,0.585); p-value: 0.018) using the Pseudo-REML method and 0.312 (95% CI: (0.104,0.519); p-value: 0.003) using Riley method. The estimated overall marginal correlation 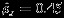 using Riley method. The results from both methods suggest that the log hazard ratio of overall-survival is significantly higher than that of the disease-free survival. The discrepancies between two methods in significance level and in width of confidence interval are consistent with what we observed in the simulation studies where Riley method tends to underestimate the standard error of the difference in pooled estimates in small sample size settings. For illustration purpose, we also calculate the estimated difference using separate univariate meta-analyses ignoring the correlation between the overall log hazard ratio with respect to overall survival and that with respect to disease-free survival. Compared to the results using Pseudo-REML method, the estimated difference δ using univariate analyses has the same point estimate but wider confidence interval. Specifically, the confidence interval is 95% CI: (0.032,0.607) and p-value is 0.030. We note that the inference using univariate meta-analyses is potentially misleading because the effect with respect to overall survival and that with respect to disease-free survival are likely to be positively correlated. Ignorance of such correlation can lead to the overestimated standard error and inflated p-value.
using Riley method. The results from both methods suggest that the log hazard ratio of overall-survival is significantly higher than that of the disease-free survival. The discrepancies between two methods in significance level and in width of confidence interval are consistent with what we observed in the simulation studies where Riley method tends to underestimate the standard error of the difference in pooled estimates in small sample size settings. For illustration purpose, we also calculate the estimated difference using separate univariate meta-analyses ignoring the correlation between the overall log hazard ratio with respect to overall survival and that with respect to disease-free survival. Compared to the results using Pseudo-REML method, the estimated difference δ using univariate analyses has the same point estimate but wider confidence interval. Specifically, the confidence interval is 95% CI: (0.032,0.607) and p-value is 0.030. We note that the inference using univariate meta-analyses is potentially misleading because the effect with respect to overall survival and that with respect to disease-free survival are likely to be positively correlated. Ignorance of such correlation can lead to the overestimated standard error and inflated p-value.
5.2. Treatment comparison for paraoxonase 1 activities on reducing coronary heart disease risk
Paraoxonase 1 (PON1) is an enzyme synthesized in the liver. PON1 has the ability to inhibit high-density lipoprotein and is cardioprotective. Studies of the relationship between PON1 activity and coronary heart disease (CHD) risk in humans have yielded inconsistent results 31. To investigate the PON–CHD relationship, Zhao et al., 32 conducted a meta-analysis combining 55 studies, which involve 9481 CHD patients and 11,148 controls. It is important to study the difference in the standard mean difference (SMD) comparing cases and controls of paraoxonase activity of PON1, denoted by Yi1, and that comparing cases and controls of arylesterase activity of PON1, denoted by Yi2
33. For illustration purpose, we consider 17 studies where both summary measures Yi1 and Yi2 are reported. As shown in the middle left panel of Table 4, the overall difference δ is estimated as −0.075 (95% CI: (−0.377,0.228); p-value: 0.627) using the Pseudo-REML method, and −0.078 (95% CI: (−0.387,0.232); p-value: 0.623) using Riley method. The estimated overall marginal correlation 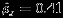 using Riley method. The results from both methods suggest there is no statistically significant differences in SMD between the paraoxonase and arylesterase activities. The results from both methods are very similar, which agree with the simulation studies with relatively large number of studies.
using Riley method. The results from both methods suggest there is no statistically significant differences in SMD between the paraoxonase and arylesterase activities. The results from both methods are very similar, which agree with the simulation studies with relatively large number of studies.
We note that many of the studies have only one of two outcomes reported. Excluding these studies in the analysis may lead to a substantial loss of efficiency. Under the MCAR assumption, both Riley method and Pseudo-REML method can be applied to all 55 studies, and the results are summarized in the middle right panel of Table 4. The overall difference δ is estimated as −0.300 (95% CI: (−0.928,0.328); p-value: 0.349) using the Pseudo-REML method and −0.281 (95% CI: (−0.688,0.126); p-value: 0.176) using Riley method. Comparing with the estimates based on the 17 studies with both endpoints reported, the estimates based on all studies have larger difference in SMD between the paraoxonase and arylesterase activities of PON 1. But none of the estimates reaches statistically significant difference. However, as suggested by the simulation studies, the confidence interval produced by Riley method tends to be too narrow, which may be corrected by the Pseudo-REML method.
5.3. Methylene tetrahydrofolate reductase gene, homocysteine, and coronary heart disease
The observed effect of blood homocysteine on CHD suggested by many observational studies is questioned by researchers because of potential confounders (e.g., smoking and blood pressure) and reverse causation (i.e., elevations in blood homocysteine may result from atherosclerosis and CHD) 34. Having a common polymorphism affecting the level of homocysteine in blood, methylene tetrahydrofolate reductase gene can be employed as an instrumental variable to adjust for the bias due to confounding or reverse causation 35. Thompson et al., 36 conducted a meta-analysis involving 66 genetic studies to estimate the unconfounded association of the homocysteine level and CHD. Comparing with the wildtype CC, the mutant genotype TT of methylene tetrahydrofolate reductase gene is associated with both risk increase of CHD and higher level of homocysteine. Let logORTT vs. CC be the log odds ratio for the association between genotype (TT vs. CC) and CHD, and let δP be the mean difference in homocysteine level between genotype TT and CC. The objective is to estimate the ratio logORTT vs CC/δP, which is the effect of one unit change in homocysteine level on logORTT vs CC. Out of the 66 studies, 18 studies reported both logORTT vs. CC and δP, while the remaining studies only reported one of the two outcomes. The estimates of logORTT vs CC/δP using the Riley method and Pseudo-REML method based on the 18 studies and the estimates based on the 66 studies are shown in the lower panels in Table 4. The Riley method produces smaller pooled estimates based on 18 studies than that based on 66 studies (0.055 vs. 0.072), while the Pseudo-REML method produces similar pooled estimates (0.076 vs. 0.070) based on 18 or 66 studies. The estimated overall marginal correlation 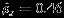 using Riley method. The difference between the pooled estimates from Riley method and those from the proposed Pseudo-REML method is due to the change in individual pooled estimates through borrowing of strength across outcomes in the Riley method, but the Pseudo-REML method does not allow this. The length of confidence interval produced by Riley method is similar to that produced by the Pseudo-REML method. The results based on all 66 studies using both methods suggest that there is a statistically significant effect of homocysteine level on CHD.
using Riley method. The difference between the pooled estimates from Riley method and those from the proposed Pseudo-REML method is due to the change in individual pooled estimates through borrowing of strength across outcomes in the Riley method, but the Pseudo-REML method does not allow this. The length of confidence interval produced by Riley method is similar to that produced by the Pseudo-REML method. The results based on all 66 studies using both methods suggest that there is a statistically significant effect of homocysteine level on CHD.
6. Discussion
In this paper, we propose a Pseudo-REML method for multivariate random-effects meta-analysis. The idea is to base the inference on a working independence model 19. Such an idea is motivated by the construction of the generalized estimating equation by Liang and Zeger 37 for the inference of marginal models in longitudinal data and the construction of the pseudo-partial likelihood function by Lin 38 for the analysis of multivariate survival data. This method is applicable when the within-study correlations are unknown, is not suffering from problems related to singularity of estimated covariance matrix, and can maintain a reasonably good inference performance in all the scenarios tested in the simulation studies. In addition, the Pseudo-REML method can be extended to multivariate meta-regression models where study-level covariates are available. More details of the regression extension can be found in the Supplemental Materials.
In this paper, we compare the performance of the REML method, Riley method, and the proposed Pseudo-REML method, and investigate their singular estimated covariance matrix problem. The REML method is the standard approach when the within-study correlations are known. However, it may suffer from the singular covariance matrix problem and can lead to poorly estimated between study correlations. The Riley method or the proposed Pseudo-REML method can be a good alternative in this situation. When within-study correlations are unknown, both Riley method and the proposed Pseudo-REML method are applicable. Riley method performs well when the estimated synthesis correlation parameter ρs is relatively small. When the estimated synthesis correlation parameter ρs is relatively large, the proposed Pseudo-REML method will be the best choice.
We note that this paper focuses on the method for meta-analysis based on summary data rather than on methods based on individual patient data (IPD). Naturally, meta-analyses based on summary data suffer from limitations such as potentially inconsistent exclusion criterion for patients across studies, and no quality assessment available based on the summary data alone 39–44. The IPD meta-analysis for pooling data from different studies should be preferred when the IPD are available; for example, see Piedbois et al., 45 and Di Leo et al., 46. In this case, the correlations between multiple outcomes would become available.
Because both the REML method and Riley method are presented in the bivariate setting 2,13, we also presented the Pseudo-REML method in the bivariate setting in this paper to keep the notation simple and convey the main idea. The Pseudo-REML method can be easily applied to MMA where more than two outcomes are analyzed. In contrast, although it is also conceptually straightforward that REML method and Riley method can be extended to MMA with more than two outcomes, we expect that these two methods will experience more severe singular estimated covariance matrix problem as the number of outcomes increases (due to the increased number of between-study correlations that require estimation). The performance of the Pseudo-REML method with more than two outcomes requires further investigation.
In this paper, we considered Pseudo-REML method when data are MCAR. One limitation of this procedure is that the Pseudo-REML method may yield biased estimates if MCAR assumption is violated, for example, in the settings of MAR or in the presence of outcome reporting bias 47 or publication bias. The current version of our Pseudo-REML method is only suitable for MCAR situations. Extension of our method to MAR and MNAR situations might be possible. For example, if publication bias is present, statistical methods such as ‘Trim and Fill’ method may be used to impute the missing studies and then incorporated into the proposed Pseudo-REML 48. The development of the Pseudo-REML under MAR and MNAR settings and the finite sample performance of regression extension of our method is currently under investigation and will be reported elsewhere.
Acknowledgments
Yong Chen was supported by grant number R03HS022900 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. Whilst undertaking this work, Richard Riley was supported by funding from an MRC Methodology Research Program Grant (MR/J013595/1). The authors want to thank Professor Geert Molenberghs for helpful comments and pointing out several key references in composite likelihoods. The authors are especially grateful to an anonymous reviewer who identified that our original REML estimation procedure could be improved. Yong Chen is grateful for the general advices from Dr. Shengfeng Cheng.
Appendix
Appendix A
Derivation of the asymptotic covariance formula for the maximum pseudolikelihood estimator
Denote 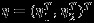 . By Taylor expansion of ∂Lp(η)/∂(η) around η, we have
. By Taylor expansion of ∂Lp(η)/∂(η) around η, we have
| A 1 |
Therefore, we have
It is easy to show that 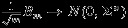 where
where
as m→∞. Note that the off-diagonal terms in Σ* account for the fact that the multivariate outcomes may be dependent. Finally, the asymptotic distribution is immediately followed by Slutskys theorem and recalling equation (A.2).
Derivation of the asymptotic variance formula for the maximum pseudolikelihood estimator under the missing data scenario
Denote 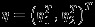 . By Taylor expansion of
. By Taylor expansion of 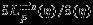 around η, we have
around η, we have
| A 2 |
Therefore, we have
It is easy to show that 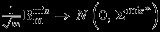 where
where
where m1/m→r1,(m1+m2)/m→r2, and (m1+m3)/m→r3. The asymptotic distribution is immediately followed by Slutsky's theorem and recalling equation (A.2).
Estimation of the covariance matrix under missing data scenario Σmis
The information matrices 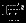 ,
, 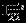 , and
, and 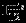 can be empirically estimated as
can be empirically estimated as
 |
where
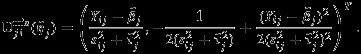 |
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's web site.
References
- 1.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 2.Van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Statistics in Medicine. 2002;21(4):589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- 3.Jones A, Riley R, Williamson P, Whitehead A. Meta-analysis of individual patient data versus aggregate data from longitudinal clinical trials. Clinical Trials. 2009;6(1):16–27. doi: 10.1177/1740774508100984. [DOI] [PubMed] [Google Scholar]
- 4.Hand D. Evaluating diagnostic tests: the area under the roc curve and the balance of errors. Statistics in Medicine. 2010;29(14):1502–1510. doi: 10.1002/sim.3859. [DOI] [PubMed] [Google Scholar]
- 5.Jackson D, Riley R, White I. Multivariate meta-analysis: potential and promise. Statistics in Medicine. 2011;30(20):2481–2498. doi: 10.1002/sim.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borenstein M, Hedges L, Higgins J, Rothstein H. Introductionto Meta-Analysis. The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, United Kingdom: Wiley Online Library; 2009. [Google Scholar]
- 7.Hartung J, Knapp G, Sinha BK. Statistical Meta-Analysis with Applications. Hoboken, New Jersey, USA: Wiley-Interscience; 2011. vol. 738. [Google Scholar]
- 8.Daniels MJ, Hughes MD. Meta-analysis for the evaluation of potential surrogate markers. Statistics in Medicine. 1997;16(17):1965–1982. doi: 10.1002/(sici)1097-0258(19970915)16:17<1965::aid-sim630>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Berkey C, Antczak-Bouckoms A, Hoaglin D, Mosteller E, Pihlstrom B. Multiple-outcomes meta-analysis of treatments for periodontal disease. Journal of Dental Research. 1995;74(4):1030–1039. doi: 10.1177/00220345950740040201. [DOI] [PubMed] [Google Scholar]
- 10.Nam I, Mengersen K, Garthwaite P. Multivariate meta-analysis. Statistics in Medicine. 2003;22(14):2309–2333. doi: 10.1002/sim.1410. [DOI] [PubMed] [Google Scholar]
- 11.Wei Y, Higgins J. Estimating within-study covariances in multivariate meta-analysis with multiple outcomes. Statistics in Medicine. 2013;32(7):1191–1205. doi: 10.1002/sim.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riley R, Abrams K, Sutton A, Lambert P, Thompson J. Bivariate random-effects meta-analysis and the estimation of between-study correlation. BMC Medical Research Methodology. 2007;7(1):15. doi: 10.1186/1471-2288-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riley R, Thompson J, Abrams K. An alternative model for bivariate random-effects meta-analysis when the within-study correlations are unknown. Biostatistics. 2008;9(1):172–186. doi: 10.1093/biostatistics/kxm023. [DOI] [PubMed] [Google Scholar]
- 14.Lindsay B. Composite likelihood methods. Contemporary Mathematics. 1988;80(1):221–39. [Google Scholar]
- 15.Cox D, Reid N. A note on pseudolikelihood constructed from marginal densities. Biometrika. 2004;91(3):729–737. [Google Scholar]
- 16.Varin C, Reid N, Firth D. An overview of composite likelihood methods. Statistica Sinica. 2011;21(1):5–42. [Google Scholar]
- 17.Kent JT. Robust properties of likelihood ratio tests. Biometrika. 1982;69(1):19–27. [Google Scholar]
- 18.Molenberghs G, Verbeke G. Models for Discrete Longitudinal Data. New York, NY 10013, USA: Springer; 2005. 2005. [Google Scholar]
- 19.Chandler R, Bate S. Inference for clustered data using the independence loglikelihood. Biometrika. 2007;94(1):167–183. [Google Scholar]
- 20.Huber P. 1967;1:221–33. University of California, Berkeley, CA, USA Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability The behavior of maximum likelihood estimates under nonstandard conditions, Vol. [Google Scholar]
- 21.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica: Journal of the Econometric Society. 1980;48:817–838. [Google Scholar]
- 22.White I. Multivariate random-effects meta-regression: updates to mvmeta. Stata Journal. 2011;11(2):255–270. [Google Scholar]
- 23.Gasparrini A, Gasparrini MA. Package mvmeta. 2014.
- 24.Riley R, Abrams K, Lambert P, Sutton A, Thompson J. An evaluation of bivariate random-effects meta-analysis for the joint synthesis of two correlated outcomes. Statistics in Medicine. 2007;26(1):78–97. doi: 10.1002/sim.2524. [DOI] [PubMed] [Google Scholar]
- 25.Little RJ, Rubin DB. Statistical Analysis with Missing Data. New York: Wiley; 1987. Vol. 4. [Google Scholar]
- 26.Sohn S. Multivariate meta analysis with potentially correlated marketing study results. Naval Research Logistics (NRL) 2000;47(6):500–510. [Google Scholar]
- 27.Riley R. Multivariate meta-analysis: the effect of ignoring within-study correlation. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2009;172(4):789–811. [Google Scholar]
- 28.Baade P, Youlden D, Krnjacki L. International epidemiology of prostate cancer: geographical distribution and secular trends. Molecular Nutrition & Food Research. 2009;53(2):171–184. doi: 10.1002/mnfr.200700511. [DOI] [PubMed] [Google Scholar]
- 29.Sasse A, Sasse E, Carvalho A, Macedo L. Androgenic suppression combined with radiotherapy for the treatment of prostate adenocarcinoma: a systematic review. BMC Cancer. 2012;12(1):1–11. doi: 10.1186/1471-2407-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine. 1998;17(24):2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 31.Getz G, Reardon C. Paraoxonase, a cardioprotective enzyme: continuing issues. Current Opinion in Lipidology. 2004;15(3):261–267. doi: 10.1097/00041433-200406000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Ma Y, Fang Y, Liu L, Wu S, Fu D, Wang X. Association between pon1 activity and coronary heart disease risk: a meta-analysis based on 43 studies. Molecular Genetics and Metabolism. 2011;105:141–148. doi: 10.1016/j.ymgme.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Epstein FH, Diaz MN, Frei B, Vita JA, Keaney JF,Jr. Antioxidants and atherosclerotic heart disease. New England Journal of Medicine. 1997;337(6):408–416. doi: 10.1056/NEJM199708073370607. [DOI] [PubMed] [Google Scholar]
- 34.Sterne JA, Egger M, Smith GD. Investigating and dealing with publication and other biases in meta-analysis. Bmj. 2001;323(7304):101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. Journal of the American Statistical Association. 1996;91(434):444–455. [Google Scholar]
- 36.Thompson JR, Minelli C, Abrams KR, Tobin MD, Riley RD. Meta-analysis of genetic studies using mendelian randomization–a multivariate approach. Statistics in Medicine. 2005;24(14):2241–2254. doi: 10.1002/sim.2100. [DOI] [PubMed] [Google Scholar]
- 37.Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 38.Lin DY. Cox regression analysis of multivariate failure time data: the marginal approach. Statistics in Medicine. 1994;13(21):2233–2247. doi: 10.1002/sim.4780132105. [DOI] [PubMed] [Google Scholar]
- 39.Turner R, Omar R, Yang M, Goldstein H, Thompson S. A multilevel model framework for meta-analysis of clinical trials with binary outcomes. Statistics in Medicine. 2000;19:3417–3432. doi: 10.1002/1097-0258(20001230)19:24<3417::aid-sim614>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 40.Higgins J, Whitehead A, Turner R, Omar R, Thompson S. Meta-analysis of continuous outcome data from individual patients. Statistics in Medicine. 2001;20(15):2219–2241. doi: 10.1002/sim.918. [DOI] [PubMed] [Google Scholar]
- 41.Piedbois P, Buyse M. Meta-analyses based on abstracted data: a step in the right direction, but only a first step. Journal of Clinical Oncology. 2004;22(19):3839–3841. doi: 10.1200/JCO.2004.06.924. [DOI] [PubMed] [Google Scholar]
- 42.Smith C, Williamson P, Marson A. Investigating heterogeneity in an individual patient data meta-analysis of time to event outcomes. Statistics in Medicine. 2005;24(9):1307–1319. doi: 10.1002/sim.2050. [DOI] [PubMed] [Google Scholar]
- 43.Clarke M, Stewart L, Tierney J, Williamson P. Individual patient data meta-analyses compared with meta-analyses based on aggregate data. The Cochrane Library. 2008 ) [Google Scholar]
- 44.Riley R, Lambert P, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. British Medical Journal. 2010;340(7745):521–525. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 45.Piedbois P, Buyse M. Meta-analysis based on individual patient data: example of advanced colorectal cancer. Recherche en Soins Infirmiers. 2010;101:25–28. [PubMed] [Google Scholar]
- 46.Di Leo A, Desmedt C, Bartlett JM, Piette F, Ejlertsen B, Pritchard KI, Larsimont D, Poole C, Isola J, Earl H, Mouridsen H, O'Malley FP, Cardoso F, Tanner M, Munro A, Twelves CJ, Sotiriou C, Shepherd L, Cameron D, Piccart MJ, Buyse M, HER2/TOP2A Meta-analysis Study Group Her2 and top2a as predictive markers for anthracycline-containing chemotherapy regimens as adjuvant treatment of breast cancer: a meta-analysis of individual patient data. The Lancet Oncology. 2011;12(12):1134–1142. doi: 10.1016/S1470-2045(11)70231-5. [DOI] [PubMed] [Google Scholar]
- 47.Rothstein H, Sutton A, Borenstein M. Publication Bias in Meta-Analysis. The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, United Kingdom: Wiley Online Library; 2005. [Google Scholar]
- 48.Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


