Abstract
Background
The etiology of Benign Prostatic Hyperplasia (BPH), a common among aged men, is not fully understood, however, in addition to androgens and aging, chronic ischemia has been proposed to contribute. Using an established rat model, we investigated whether chronic ischemia alters the structural and functional properties of the ventral rat prostate, and whether phosphodiesterase type 5 (PDE5) inhibitor (tadalafil) may have a protective action.
Methods
Adult male Sprague-Dawley rats were divided into control, arterial endothelial injury (AI), and AI with tadalafil treatment (AI-tadalafil) groups. AI and AI-tadalafil groups underwent endothelial injury of the iliac arteries and received a 2% cholesterol diet following AI. AI-tadalafil rats were treated with tadalafil (2 mg/kg/day) orally for 8 weeks after AI. The control group received a regular diet. After 8 weeks, animals were sacrificed, and pharmacological and morphological studies on prostate tissues were performed.
Results
Iliac arteries from AI rats displayed neo-intimal formation and luminal occlusion, an effect that was not prevented by tadalafil treatment. In the AI group, there was an obvious epithelial atrophy and a statistically significant increase in collagen fibers compared with the controls. Immunohistochemically, there was an up-regulation of smooth muscle α-actin (SMA). Contractile responses of prostate strips to KCl, electrical field stimulation (EFS), and phenylephrine (PE) were significantly higher after AI than in controls. Chronic treatment with tadalafil prevented the increase in contractile responses in ischemic tissue, and decreased the collagen deposition compared with the AI group.
Conclusions
In this rat model, chronic pelvic ischemia caused distinct functional and morphological changes in the prostate. Prostatic tissue from ischemic animals showed an increased contractile response to electrical and pharmacological stimulation, an increase in SMA, and an increased deposition of collagen. All these changes could be prevented by treatment with the PDE5 inhibitor, tadalafil, suggesting an involvement of cyclic guanosine monophosphate (cGMP). Prostate 75:233–241, 2015. © 2014 The Authors. The Prostate Published by Wiley Periodicals, Inc.
Keywords: arterial occlusive disease, benign prostatic hyperplasia, chronic prostate ischemia, phosphodiesterase type 5 inhibitor
Introduction
Benign Prostatic Hyperplasia (BPH) is a common medical problem among aged men. Its etiology is not fully understood, but age, hormones, and epithelial–mesenchymal interactions are all believed to be contributing factors to the pathogenesis [1–4]. It is reasonable to expect a relation between voiding lower urinary tract symptoms (LUTS) and prostate size, and population-based studies have indeed demonstrated modest correlations between benign prostatic enlargement, bladder outflow obstruction (BOO), and LUTS [5]. However, even for voiding LUTS, evidence for a direct link is far from convincing, and it is now recognized that such symptoms do not reliably reflect the underlying vesico-urethral pathology. A strong association has been demonstrated between BPH, LUTS, and risk factors for atherosclerosis such as hypertension, diabetes, and heart disease [4].
Vascular endothelial dysfunction occurs with aging and is an independent risk factor for the development of atherosclerosis and hypertension [6,7]. The abdominal aorta and its branches, especially the bifurcation of the iliac arteries, are particularly vulnerable to atherosclerotic lesions. Since the vascular supply to the human genitourinary tract, including bladder, prostate, urethra, and penis, is primarily derived from the iliac arteries, atherosclerotic obstructive changes distal to the aortic bifurcation will have consequences for the distal vasculature and for LUT blood flow. In fact, Berger et al., [8] using contrast enhanced color Doppler ultrasonography, found significantly reduced perfusion of the LUT in men at high risk for LUTS. In men with diabetes or peripheral arterial occlusive disease, perfusion in the transition zone of the prostate was significantly reduced and LUTS was significantly worse, compared to healthy controls. This implies that arterial occlusive disease (atherosclerosis) and concomitant reduced perfusion of the prostate may have impact on prostate enlargement (static component) and/or prostatic smooth muscle tone (dynamic component).
The presence of LUTS in men has traditionally been thought to be related to the growth of the prostate, with subsequent urethral compression and obstruction, and secondary bladder overactivity. However, based on a rabbit model of chronic lower body ischemia, it has been suggested that tissue fibrosis, along with cellular proliferation/prostatic enlargement and smooth muscle hypercontractility, may act independently or in combination to promote male LUTS [9,10].
Phosphodiesterase type 5 (PDE5) inhibitors, such as tadalafil, sildenafil, and vardenafil have been shown to be effective for treatment of LUTS associated with BPH [11–14]. These drugs are known to increase the amount of cyclic guanosine monophosphate (cGMP) in, the smooth muscle of the corpus cavernosum, prostate, and bladder. However, the mechanisms of action when relieving LUTS have not been established [15,16], and how an increase in cGMP may affect prostatic smooth muscle tone under chronic ischemic conditions is not known, even if it has been shown that in rabbit prostate, chronic ischemia decreases relaxant responses to electrical stimulation of nerves, probably related to reduced nitric oxide (NO) release [9].
We have utilized a previously described rat model of chronic lower body ischemia [17] to investigate whether chronic ischemia alters the structural and functional properties of rat prostate, and whether ischemic effects can be prevented by tadalafil treatment.
Materials and Methods
The experimental protocol, which complied with set guidelines for animal experiments, was reviewed and approved by the Animal Care and Use Committee, Wake Forest University.
Experimental Design
Adult male Sprague-Dawley rats (440–500 g) were divided into arterial endothelial injury (AI), AI treated with tadalafil (AI-tadalafil) and age-matched control groups. The AI and AI-tadalafil groups underwent balloon endothelial injury of the iliac arteries and received a 2% cholesterol diet for 8 weeks (AI: n = 11, AI-tadalafil: n = 8). The AI-tadalafil group was treated with tadalafil orally (Cialis® tablets, Lilly) once daily at a dose of 2 mg/kg for 8 weeks. The dose was chosen based on published experiences with the drug [18]. The crushed Cialis® tablets, which were purchased from Wake Forest Baptist Hospital, were dissolved in water and placed in the oropharynx with a syringe. A third group receiving a regular diet for 8 weeks was used as an age-match control group (n = 8).
Rats from each group were euthanized with CO2 inhalation and thoracotomy. The whole rat ventral prostate was obtained by an incision along the midline of the lower abdomen that exposed the urogenital tract. Immediately following excision, one part of ventral prostate was used for organ bath studies, and another part was processed for immunohistochemical and histological examination. In addition, vessels from aorta to femoral arteries were removed and used for histological examination.
Arterial Balloon Endothelial Injury (AI) of the Iliac Arteries
The procedure for producing AI has been described previously [17]. Briefly, the animals were anesthetized with 3% isoflurane, and a Fogarty arterial embolectomy catheter (E-060-2F) from Edwards Lifesciences LLC (Irvine, CA) was passed through the femoral artery into the common iliac artery. The balloon was inflated with air and subsequently withdrawn from the common iliac artery to the femoral artery, a maneuver repeated 10 times on each side.
Organ Bath Experiments
The dissected prostate lobes were placed in a petri dish containing Krebs–Henseleit solution (in mM: NaCl 118.1, KCl 4.69, KH2PO4 1.2, NaHCO3 25.0, glucose 11.7, MgSO4 0.5, and CaCl2 2.5), and the excess fat and connective tissue was removed to facilitate drug distribution throughout the tissue. Tissues were then mounted in 15-ml water-jacketed glass organ baths containing Krebs–Henseleit solution, maintained at 37°C, and bubbled with 5% CO2 in O2. One end of the prostate tissue was attached to a static platinum hook embedded in the tissue holder. The other end of the prostate was attached to an isometric force transducer (Danish Myo Technology). During the course of the experiment, the resting tension of the preparations was maintained at 0.5 g. Pilot studies showed this to be the optimal tension range for contractile responses over the duration of the experiment. Before experimentation, the tissue preparations were equilibrated for 30 min. The bath solution was replaced every 15 min during the equilibration period and as needed during experiments to avoid any sporadic disturbances caused by frothing from tissue secretions. After equilibration, the Krebs–Henseleit solution was replaced with a high K+ (60 mM) Krebs–Henseleit solution to measure the contractile potential of the preparation. In order to compare maximum contractile responses, prostate tissues from the different groups were contracted with 10−5 M phenylephrine (PE). Nerves within the prostatic smooth muscle were electrically stimulated (electrical field stimulation: EFS) via two parallel platinum electrodes incorporated in the tissue holder, which was connected to a Grass S88 stimulator. Frequency-response curves to EFS were constructed, each stimulation lasting 10 sec at increasing frequencies (0.5 ms pulse duration, 60 V, 1, 2, 4, 8, 16, 32 Hz). At the end of each experiment the sodium channel blocker tetrodotoxin (1 µM) was added.
After the 30-min rest period, concentration-response curves to PE were constructed using increasing concentrations of PE (10−8–10−4 M).
After experimentation, prostates were removed from the organ baths, blotted dry, and weighed. All tissue responses were normalized to gram of tissue weight. Drug concentrations are expressed as final concentrations in the bath and all drugs were dissolved and diluted in distilled water. Drugs and chemicals were obtained from Sigma (St. Louis, MO).
Histology and Morphometry
The iliac arteries and the bilateral lobes of the ventral prostate from each group were placed in 10% neutral-buffered formalin. A complete longitudinal section was prepared from each lobe. Standard procedures of paraffin embedding, sectioning (5 µm) and staining with hematoxylin and eosin (H&E) and Masson's trichrome stain were carried out. In the morphometric analysis, histology pictures of the prostate gland were visualized with a light microscope under low-power (40×) views. From each rat, five sections were selected randomly. In each section, the two thinnest and two thickest parts of the trabecular wall were measured, and the mean of these measurements were used for comparisons.
Computer assisted histomorphometric analysis of H&E stained common iliac arteries and Masson's trichrome stained prostate tissues, was performed using image analysis software (Image-Pro Express) and a Leica microscope. Arterial wall thickness in each common iliac artery from all animals was determined by averaging arterial wall thickness from four positions.
The percentage of collagen in ventral prostate is based on the area calculation of smooth muscle (red stained) and connective tissue (blue stained) in randomly selected four high power fields from each animal. The percentage of collagen in ventral prostate was calculated for every high power field as the sum of the blue stained areas divided by the sum of all red and blue stained areas. Slides were examined by a single investigator.
Immunohistochemistry
Sections were incubated with 5% skimmed milk. Then, sections were incubated with the primary antibody to α-SMA (Ab 48508, mouse monoclonal, 1:50), for 60 minutes at room temperature. Following primary antibody incubation, slides were treated with Texas red-conjugated goat anti-mouse secondary antibody (Vector, CA). Then, proteins were visualized. Nuclear staining was performed with 4′,6-diamidino-2-phenylindole (DAPI, 1 µg/ml, Sigma–Aldrich, St. Louis, MO).
Analysis of data
All data analyses were carried out using GraphPad Prism (v 5.0). Results are expressed as the mean ± SEM, where the value of n represents the number of experimental animals used. In all data analyses, P ≤ 0.05 was considered significant. All data were analyzed by one-way ANOVA with a Tukey post-test for multiple comparisons where appropriate. P-values were the probability of a significant difference in mean values. The peak force (g/g tissue) of EFS- or PE- and KCl-induced contractile responses was measured at each frequency or concentration in each group. Graphs of both mean frequency-response and mean concentration-response data were constructed. In frequency-response and concentration-response graphs, the mean contractile responses of control group were compared with AI and AI-tadalafil groups.
Results
Among a total of 30 rats undergoing AI, five (16.6%) animals died or were euthanized postoperatively due to vascular bleeding into retroperitoneum or aggravation of the general condition, and additionally six rats were excluded from study because of macroscopic hematuria and dribbling voiding.
Body and Prostate Wet Weights
All surviving animals were in good condition at the end of the experiments; no side effects were observed in the tadalafil treated group. Eight weeks following AI, the body and prostate wet weights were not significantly different among the three groups (Table I). All rats that received a 2% cholesterol diet showed a marked enlarged fatty liver at necropsy.
Table I.
Body and Prostate Weights in Control, AI, and AI-Tadalafil Groups
| Group | n | Body weight (g) | Prostate weight (g) |
|---|---|---|---|
| Control | 8 | 584.62 ± 16.05 | 1.39 ± 0.14 |
| AI | 11 | 605.36 ± 10.77 | 1.88 ± 0.07 |
| AI-tadalafil | 8 | 591.25 ± 19.52 | 1.03 ± 0.06 |
Wall Thickness of the Common Iliac Arteries
In order to demonstrate obstruction of the common iliac arteries from the AI and AI-tadalafil groups, H&E staining was performed. An obvious arterial wall thickening with formation of a neo-intima compared with the control group was found (Fig. 1; data not shown).
Fig 1.
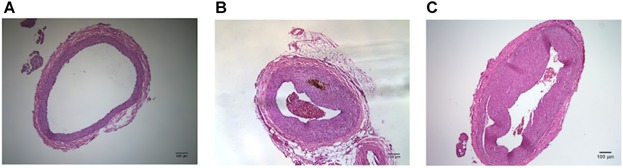
Demonstration of obstruction of the common iliac arteries in vessels from control (A), AI (B), and AI-tadalafil (C) groups by using H&E staining. Compared to the controls, the AI group showed an obvious arterial wall thickening with formation of neo-intima, and effect that was not prevented by tadalafil treatment.
Contractile Responses to Drugs and Electrical Stimulation
The functional changes induced by chronic ischemia were characterized by studying the responses to different contractile stimuli.
To measure the contractile potential of the preparations, all ventral prostate strips were initially exposed to 60 mM KCl. In the AI group, the mean contractile response to 60 mM KCl was significantly higher than in the control group (P < 0.01; Fig. 2A). The AI-tadalafil group had a significantly lower mean contractile response than the AI group (P < 0.01).
Fig 2.
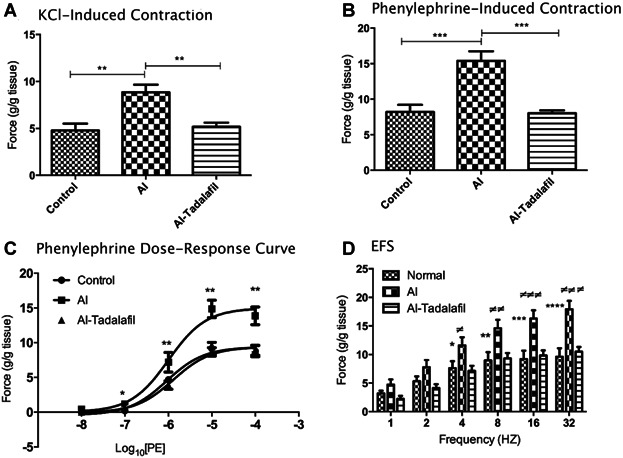
The functional changes induced by chronic ischemia induced by different contractile stimuli and the effects of tadalafil. Contractile responses were induced by 60 mM KCl (panel A; n = 8 for all groups) and phenylephrine 10−5 M (panel B; n = 8 for all groups). Concentration-response curves to phenylephrine (panel C), and frequency-dependent responses to EFS (panel D) were determined in control, AI, and AI-tadalafil groups (control n = 8, AI n = 11, and AI-tadalafil n = 8). *P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.05 AI-tadalafil versus AI, ##P < 0.01 AI-tadalafil versus AI, ###P < 0.001 AI-tadalafil versus AI, ####P < 0.0001 AI-tadalafil versus AI.
Cumulative concentration-responses curves showed that in the AI group, the contractile responses to PE at concentrations 10−7–10−4 M were significantly higher than in the other groups (Fig. 2B). However, the concentrations inducing 50% contraction (EC50), in control, AI, and AI-tadalafil groups (−6.04 ± 0.13, −5.99 ± 0.15 and −5.88 ± 0.11, respectively) were not significantly different. In the AI group, prostate strips treated with 10−5 M of PE (producing maximum contraction; g/g tissue) showed a significantly higher contraction amplitude (14.7 ± 1.47) compared with the control group (7.67 ± 0.96 (P < 0.001). Contraction in the AI-tadalafil group (8.02 ± 2.83) was significantly lower than in the AI group (P < 0.001; Fig. 2C), but not different from the control group.
The contractile responses induced by EFS in the AI ventral prostate strips were significantly higher than those in the control ventral prostate strips at frequencies 8, 16, and 32 Hz (P < 0.05, P < 0.01, P < 0.001, respectively; Fig. 2D). In the AI-tadalafil group, the contractile responses to EFS was significantly lower than those in the AI group at 2, 4, 8, 16, and 32 Hz (P < 0.05, P < 0.05, P < 0.05, P < 0.01, P < 0.01, respectively; Fig. 2D). All data showed no significant difference between control and AI-tadalafil group. The sodium channel blocker, tetrodotoxin (1 µM), abolished the EFS responses in all groups (data not shown).
Effects on Trabecular Glandular Tissue
H&E staining was used to assess the morphological changes in the prostate caused by chronic ischemia. A thinning of the wall of the trabecular glandular tissue in the AI prostate was demonstrated (Fig. 3). The average wall thickness in the AI group was significantly lower than that of the other groups (P < 0.001, Fig. 3G).
Fig 3.
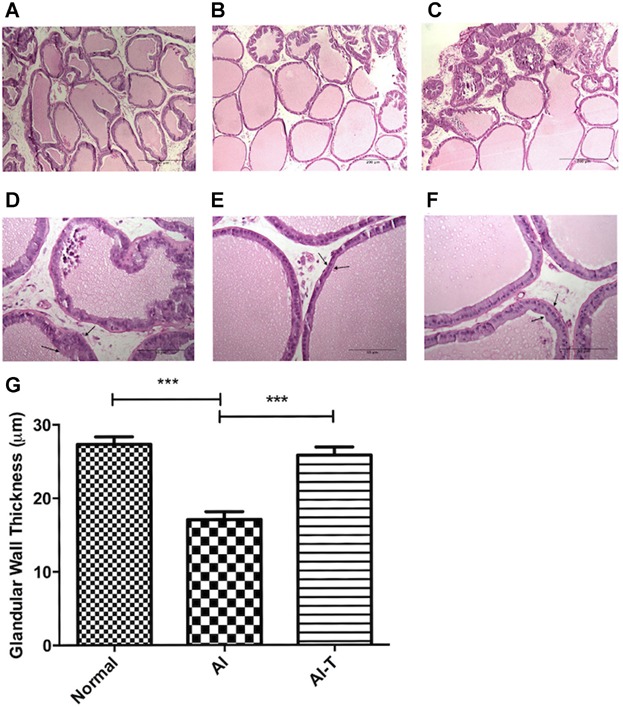
Average glandular wall thickness was assessed by H&E staining of the ventral prostate (upper panel: 100× (original magnification), scale bar represents 200 µm, lower panel: 400× (original magnification), scale bar represents 50 µm) in control (panels A,D), AI (panels B, E) and AI-tadalafil (panels C, F) groups. Arrow represents glandular wall. In panel G the average glandular wall thickness is quantified in the control (Normal) (n = 8), AI (n = 11), and AI-tadalafil (n = 8) groups. ***P < 0.001.
Effects on Stromal Components
Masson's trichrome differentially stains stromal components and, hence, is useful in distinguishing prostate smooth muscle cells (SMC) from collagen fibers and other stromal cell (SC) types. Analysis of control prostate stroma showed a mixture of red-staining SMC and blue-staining collagen fibers (Fig. 4). There was a significantly increased percentage of collagen in the stromal layer in the AI group (16.9 ± 1.3%) compared with the control (8.91 ± 0.9%, P < 0.05) and AI-tadalafil groups (10.99 ± 1.9%, P < 0.05) (Fig. 4G).
Fig 4.
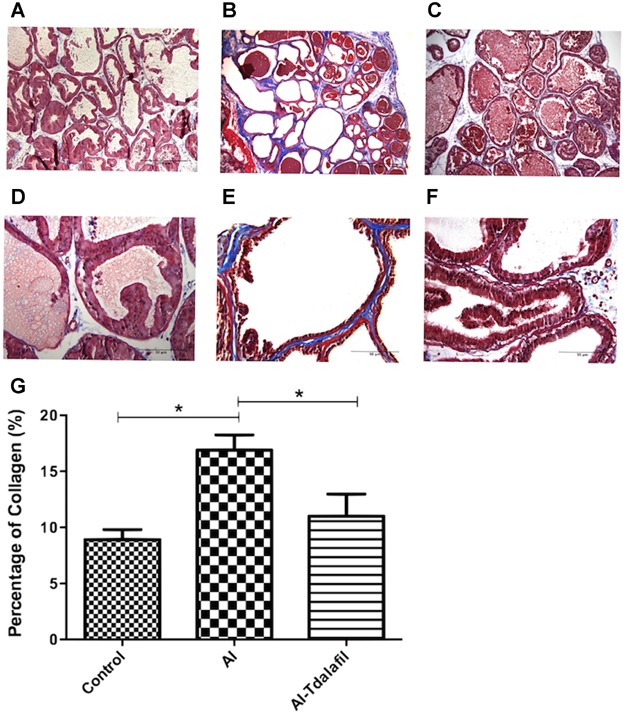
Demonstration and quantification of the amounts of collagen (blue) and stroma (red) in the rat ventral prostate using Masson's Trichrome staining in animal of the control (A, D), AI (B, E), and AI-tadalafil (C, F) groups (upper panel: 100× (original magnification), scale bar represents 200 µm, lower panel: 400× (original magnification), scale bar represents 50 µm). Panel G shows percentage of collagen in ventral prostate glands (*P < 0.05).
Effects on the Expression of Smooth Muscle α-Actin
Antibodies to smooth muscle α-actin (SMA) are commonly used to study smooth muscle differentiation in normal and pathological conditions. Stroma from control prostate gland showed low levels of SMA expression in ventral prostate tissue (Fig. 5A and D). As suggested by the initial trichrome staining, there was a change in expression of the stromal components throughout ischemic prostatic stroma. The stroma in AI group exhibited an increase in SMA staining (Fig. 5B and E) that directly correlated with red staining regions observed with Masson's trichrome staining (Fig. 4B and E).
Fig 5.
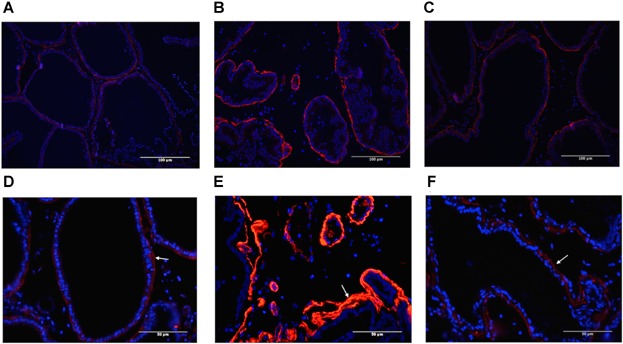
Immunodistribution of smooth muscle α-actin (SMA) in ventral prostate of control (A, D), AI (B, E), and AI-tadalafil (C, F) groups, using SMA antibodies (upper panel: 200×, scale bar represents 100 µm, lower panel: 400× (original magnification), scale bar represents 50 µm). The SMA staining in the fibromuscular stroma is conspicuous, in contrast to the weak staining in epithelium.
Discussion
Atherosclerosis-induced arterial insufficiency is a common clinical problem in the elderly [19]. Studies have shown that there is an association between the development of BPH and manifestations of atherosclerotic disease such as noninsulin-dependent diabetes mellitus, hypertension, or dyslipidemia, suggesting that BPH is a component of the metabolic syndrome and that the underlying cause of BPH might be systemic rather than local [20,21]. It has been shown both in the rabbit and the rat that balloon-induced endothelial injury of the iliac arteries combined with a high cholesterol diet for 8 weeks induces arterial occlusive disease and consequent lower body ischemia [9,17], allowing studies of the effect of chronic ischemia on prostatic structure and function. In the rabbit, chronic prostate ischemia caused stromal fibrosis and glandular cystic atrophy [9,10]. Despite this, chronic ischemia significantly increased the contractile response of isolated prostatic tissue to EFS and to PE. We could found these findings in the rat and confirm that in the AI group, there was an obvious epithelial atrophy and a statistically significant increase in collagen fibers compared with the controls. The average trabecular wall thickness in the AI group was significantly lower than in control, but there was an increase in SMA staining in the AI group. Contractile responses of prostate strips to KCl, EFS, and PE were significantly higher after AI than in the controls. We also found that chronic treatment with tadalafil prevented the increase in the contractile responses to KCl, EFS, and PE, and the increase of the collagen/muscle ratio.
Atherosclerosis is well-known to be an inflammatory process involving a number of pro-inflammatory cytokines, and it is considered that there is a state of heightened oxidative stress characterized by lipid and protein oxidation in the vascular wall. Azadzoi et al. [22] found that the mechanism of increased prostate tissue contractions in ischemia involved oxidative modifications of cellular and subcellular elements. They found that cultured human prostate SMC, epithelial cells (EC), and SC were highly sensitive to hypoxic and oxidative stress conditions exhibiting differential reactions, and also that DNA damage was a widespread phenomenon. It was suggested that increased prostate contractions in ischemia and oxidative stress appeared to involve free radical incursion of SMC, EC, and SC, impairment of cellular antioxidant defense system and subsequent accumulation of oxidative and nitrosative cytotoxic elements. It has been reported that chronic treatment with tadalafil has an anti-inflammatory effect on endothelial cells [23] and such an effect may contribute to its effect on the prostate. This was further supported by the finding that tadalafil was able to blunt inflammatory responses induced by metabolic as well as inflammatory stimuli in human myofibroblast prostatic cells [24]. The role of the NO pathway in the prostate and its relation to smooth muscle tone and LUTS have been discussed by previous authors [25–29]. Several components of the prostate are endowed with NO synthase-containing nerves, for example, the fibromuscular stroma, the glandular epithelium, and the prostatic vessels [29,30]. Since NO has a relaxant effect on prostate smooth muscle and prostatic vessels, lack of NO may contribute both to increased muscle tone and reduced blood flow to the gland. In the bladder, it has been demonstrated that lower body ischemia decreases the expression of both neuronal and endothelial NO synthase [31]. Such an effect can be assumed to be exerted also in the prostate, leading to lack of NO and cGMP, thus creating a basis for treatment with PDE5 inhibitors [32].
Interestingly, chronic ischemia, as studied in the same rat model, decreased bladder contractility [17,33]. Also in the bladder, chronic ischemia induces oxidative stress and elevation of proinflammatory cytokines and other inflammatory mediators [31,34]. It seems reasonable to assume the both the degree of ischemia and its duration should influence the results. Since the bladder [31] and the prostate (present study) were exposed to the same reduction of blood flow (tissues were taken from the same animals), it seems that the time course of the tissue reaction to the reduced blood flow differed. The reasons for this difference remain to be established.
Conclusions
Prostatic tissue from rats exposed to chronic ischemia showed an increased contractile response to electrical and pharmacological stimulation, an increase in SMA, and an increased deposition of collagen. All these changes could be prevented by treatment with the PDE5 inhibitor, tadalafil, suggesting an involvement of cGMP.
References
- 1.Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011;8(1):29–41. doi: 10.1038/nrurol.2010.207. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: Past, present and future. Differentiation. 2011;82(4–5):184–199. doi: 10.1016/j.diff.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schauer IG, Rowley DR. The functional role of reactive stroma in benign prostatic hyperplasia. Differentiation. 2011;82(4–5):200–210. doi: 10.1016/j.diff.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vignozzi L, Rastrelli G, Corona G, Gacci M, Forti G, Maggi M. Benign prostatic hyperplasia: A new metabolic disease. J Endocrinol Invest. 2014 doi: 10.1007/s40618-014-0051-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Girman CJ, Jacobsen SJ, Guess HA, Oesterling JE, Chute CG, Panser LA, Lieber MM. Natural history of prostatism: Relationship among symptoms, prostate volume and peak urinary flow rate. J Urol. 1995;153(5):1510–1515. doi: 10.1016/s0022-5347(01)67448-2. [DOI] [PubMed] [Google Scholar]
- 6.Higashi Y, Kihara Y, Noma K. Endothelial dysfunction and hypertension in aging. Hypertens Res. 2012;35(11):1039–1047. doi: 10.1038/hr.2012.138. [DOI] [PubMed] [Google Scholar]
- 7.Polovina MM, Potpara TS. Endothelial dysfunction in metabolic and vascular disorders. Postgrad Med. 2014;126(2):38–53. doi: 10.3810/pgm.2014.03.2739. [DOI] [PubMed] [Google Scholar]
- 8.Berger AP, Horninger W, Bektic J, Pelzer A, Spranger R, Bartsch G, Frauscher F. Vascular resistance in the prostate evaluated by colour Doppler ultrasonography: Is benign prostatic hyperplasia a vascular disease. BJU Int. 2006;98(3):587–590. doi: 10.1111/j.1464-410X.2006.06306.x. [DOI] [PubMed] [Google Scholar]
- 9.Kozlowski R, Kershen RT, Siroky MB, Krane RJ, Azadzoi KM. Chronic ischemia alters prostate structure and reactivity in rabbits. J Urol. 2001;165(3):1019–1026. [PubMed] [Google Scholar]
- 10.Azadzoi KM, Babayan RK, Kozlowski R, Siroky MB. Chronic ischemia increases prostatic smooth muscle contraction in the rabbit. J Urol. 2003;170(2 Pt 1):659–663. doi: 10.1097/01.ju.0000064923.29954.7e. [DOI] [PubMed] [Google Scholar]
- 11.Martínez-Salamanca JI, Carballido J, Eardley I, Giuliano F, Gratzke C, Rosen R, Salonia A, Stief C. Phosphodiesterase type 5 inhibitors in the management of non-neurogenic male lower urinary tract symptoms: Critical analysis of current evidence. Eur Urol. 2011;60(3):527–535. doi: 10.1016/j.eururo.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 12.Uckert S, Oelke M. Phosphodiesterase (PDE) inhibitors in the treatment of lower urinary tract dysfunction. Br J Clin Pharmacol. 2011;72(2):197–204. doi: 10.1111/j.1365-2125.2010.03828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller MS. Role of phosphodiesterase type 5 inhibitors for lower urinary tract symptoms. Ann Pharmacother. 2013;47(2):278–283. doi: 10.1345/aph.1R528. [DOI] [PubMed] [Google Scholar]
- 14.Cantrell MA, Baye J, Vouri SM. Tadalafil: A phosphodiesterase-5 inhibitor for benign prostatic hyperplasia. Pharmacotherapy. 2013;33(6):639–649. doi: 10.1002/phar.1243. [DOI] [PubMed] [Google Scholar]
- 15.Andersson KE, de Groat WC, McVary KT, Lue TF, Maggi M, Roehrborn CG, Wyndaele JJ, Melby T, Viktrup L. Tadalafil for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: Pathophysiology and mechanism(s) of action. Neurourol Urodyn. 2011;30(3):292–301. doi: 10.1002/nau.20999. [DOI] [PubMed] [Google Scholar]
- 16.Giuliano F, Ückert S, Maggi M, Birder L, Kissel J, Viktrup L. The mechanism of action of phosphodiesterase type 5 inhibitors in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Eur Urol. 2013;63(3):506–516. doi: 10.1016/j.eururo.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Nomiya M, Yamaguchi O, Andersson KE, Sagawa K, Aikawa K, Shishido K, Yanagida T, Kushida N, Yazaki J, Takahashi N. The effect of atherosclerosis-induced chronic bladder ischemia on bladder function in the rat. Neurourol Urodyn. 2012;31(1):195–200. doi: 10.1002/nau.21073. [DOI] [PubMed] [Google Scholar]
- 18.Morelli A, Sarchielli E, Comeglio P, Filippi S, Mancina R, Gacci M, Vignozzi L, Carini M, Vannelli GB, Maggi M. Phosphodiesterase type 5 expression in human and rat lower urinary tract tissues and the effect of tadalafil on prostate gland oxygenation in spontaneously hypertensive rats. J Sex Med. 2011;8(10):2746–2760. doi: 10.1111/j.1743-6109.2011.02416.x. [DOI] [PubMed] [Google Scholar]
- 19.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet. 2013;382(9901):1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 20.De Nunzio C, Aronson W, Freedland SJ, Giovannucci E, Parsons JK. The correlation between metabolic syndrome and prostatic diseases. Eur Urol. 2012;61(3):560–570. doi: 10.1016/j.eururo.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Gacci M, Corona G, Vignozzi L, Salvi M, Serni S, De Nunzio C, Tubaro A, Oelke M, Carini M, Maggi M. Metabolic syndrome and benign prostatic enlargement: A systematic review and meta-analysis. BJU Int. 2014 doi: 10.1111/bju.12728. doi: 10.1111/bju.12728[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Azadzoi K, Zhang Q, Siroky M. Increased prostate contraction in chronic ischemia: the role of oxidative stress. Neururolol Urodyn. 2014;33(2):185. [Google Scholar]
- 23.Aversa A, Greco E, Bruzziches R, Pili M, Rosano G, Spera G. Relationship between chronic tadalafil administration and improvement of endothelial function in men with erectile dysfunction: A pilot study. Int J Impot Res. 2007;19(2):200–207. doi: 10.1038/sj.ijir.3901513. [DOI] [PubMed] [Google Scholar]
- 24.Vignozzi L, Gacci M, Cellai I, Morelli A, Maneschi E, Comeglio P, Santi R, Filippi S, Sebastianelli A, Nesi G, Serni S, Carini M, Maggi M. PDE5 inhibitors blunt inflammation in human BPH: A potential mechanism of action for PDE5 inhibitors in LUTS. Prostate. 2013;73(13):1391–1402. doi: 10.1002/pros.22686. [DOI] [PubMed] [Google Scholar]
- 25.Bloch W, Klotz T, Loch C, Schmidt G, Engelmann U, Addicks K. Distribution of nitric oxide synthase implies a regulation of circulation, smooth muscle tone, and secretory function in the human prostate by nitric oxide. Prostate. 1997;33(1):1–8. doi: 10.1002/(sici)1097-0045(19970915)33:1<1::aid-pros1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 26.Klotz T, Mathers MJ, Bloch W, Nayal W, Engelmann U. Nitric oxide based influence of nitrates on micturition in patients with benign prostatic hyperplasia. Int Urol Nephrol. 1999;31(3):335–341. doi: 10.1023/a:1007174102953. [DOI] [PubMed] [Google Scholar]
- 27.Aikawa K, Yokota T, Okamura H, Yamaguchi O. Endogenous nitric oxide-mediated relaxation and nitrinergic innervation in the rabbit prostate: The changes with aging. Prostate. 2001;48(1):40–46. doi: 10.1002/pros.1079. [DOI] [PubMed] [Google Scholar]
- 28.Hedlund P. Nitric oxide/cGMP-mediated effects in the outflow region of the lower urinary tract—Is there a basis for pharmacological targeting of cGMP. World J Urol. 2005;23(6):362–367. doi: 10.1007/s00345-005-0019-1. [DOI] [PubMed] [Google Scholar]
- 29.Kedia GT, Uckert S, Jonas U, Kuczyk MA, Burchardt M. The nitric oxide pathway in the human prostate: Clinical implications in men with lower urinary tract symptoms. World J Urol. 2008;26(6):603–609. doi: 10.1007/s00345-008-0303-y. [DOI] [PubMed] [Google Scholar]
- 30.Fernandes VS, Martínez-Sáenz A, Recio P, Ribeiro AS, Sánchez A, Martínez MP, Martínez AC, García-Sacristán A, Orensanz LM, Prieto D, Hernández M. Mechanisms involved in the nitric oxide-induced vasorelaxation in porcine prostatic small arteries. Naunyn Schmiedebergs Arch Pharmacol. 2011;384(3):245–253. doi: 10.1007/s00210-011-0666-2. [DOI] [PubMed] [Google Scholar]
- 31.Nomiya M, Burmeister DM, Sawada N, Campeau L, Zarifpour M, Keys T, Peyton C, Yamaguchi O, Andersson KE. Prophylactic effect of tadalafil on bladder function in a rat model of chronic bladder ischemia. J Urol. 2013;189(2):754–761. doi: 10.1016/j.juro.2012.07.141. [DOI] [PubMed] [Google Scholar]
- 32.Lythgoe C, McVary KT. The use of PDE-5 inhibitors in the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Curr Urol Rep. 2013;14(6):585–594. doi: 10.1007/s11934-013-0373-2. [DOI] [PubMed] [Google Scholar]
- 33.Sagawa K, Aikawa K, Nomiya M, Ogawa S, Akaihata H, Takahashi N, Yamaguchi O, Kojima Y. Impaired detrusor contractility in a rat model of chronic bladder ischemia. Urology. 2013;81(6):1379.e9-14. doi: 10.1016/j.urology.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Azadzoi KM, Chen BG, Radisavljevic ZM, Siroky MB. Molecular reactions and ultrastructural damage in the chronically ischemic bladder. J Urol. 2011;186(5):2115–2122. doi: 10.1016/j.juro.2011.06.047. [DOI] [PubMed] [Google Scholar]


