Extended Data Figure 7. Model of glutathione-dependent PrfA activation.
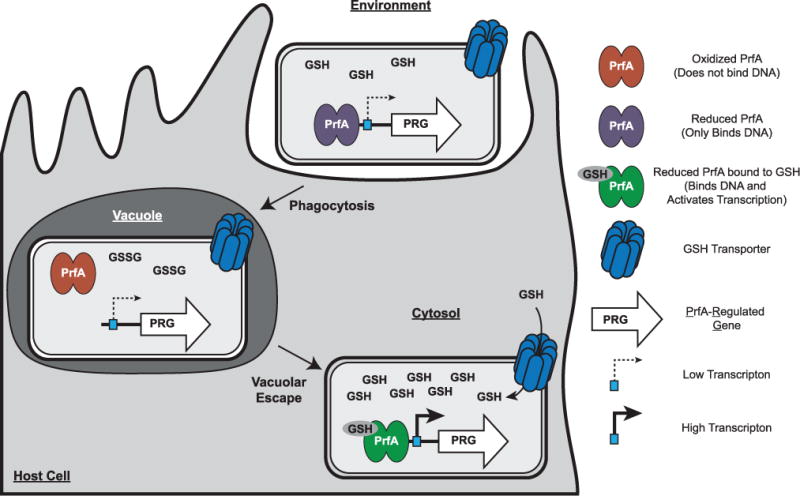
The process of infection or intercellular spread requires that L. monocytogenes inhabit an oxidizing vacuole, which may contain both reactive oxygen and nitrogen species. Upon oxidation, glutathione dimerizes to GSSG, which we have demonstrated does not bind PrfA. In addition, PrfA thiols may be reversibly oxidized, temporarily inactivating the protein by inhibiting DNA binding and leading to a downregulation of PrfA-regulated genes (PRG). L. monocytogenes could then enter the host cytosol, as PrfA activation is dispensable for vacuolar escape in vivo. The host cytosol is a highly reducing environment and upon entry into this compartment, all thiols are expected to be in the reduced form. In the absence of glutathione, it is likely that coenzyme A maintains redox homeostasis in the bacterium, as it is the most abundant LMW thiol in L. monocytogenes. Reduced glutathione could then bind PrfA and activate transcription of PRG. This two-step activation requirement may explain why the mechanism of PrfA activation has been a mystery for over two decades; the redox changes occurring during transit through a vacuole followed by replication in the highly reducing cytosol have yet to be recapitulated in vitro.
