Figure 1.
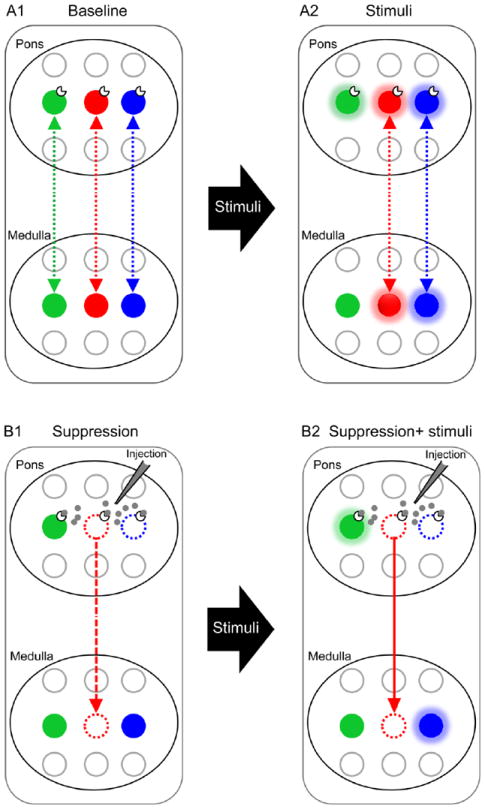
Schematic illustration of the multiscale temporal-cellular coincident fingerprinting assay for long-range functional connectivity mapping. A1: Pairs of neuronal pools in separate brain nuclei (pons and medulla) are potentially functionally connected either way (broken bidirectional arrows) if their discharge patterns in quiescent state (baseline) contain timing-specific components that are temporally correlated to one another. Distinct timing-specific correlated discharge patterns are denoted by different colors (green, red, blue). Non-correlated components are uninformative and are excluded from analysis. A2: Functional connectivity from the green pontine pool to green medullary pool cannot be ascertained if the former is excited by stimuli but not the latter. B1. To investigate whether the timing-specific and response-specific correlations among the remaining red and blue neuronal pools in pons and medullar signify functional connectivity, corresponding neuronal pools in pons are suppressed (broken open circles) by cell-specific pharmacological or other blockers. In this example, the red neuronal pool in medulla is said to likely receive excitatory drive (broken downward arrow) from its counterpart in pons, since its activity is attenuated or silenced by suppression of the latter. The same cannot be said of the blue neuronal pools, however, since the blue neuronal pool in medulla remains active after its counterpart in pons is suppressed. B2. Excitatory connectivity from red pontine pool to red medullary pool as determined in B1 is confirmed (solid downward arrow) if the response of the red medullary pool to afferent stimulation is abolished after suppression of the red pontine pool. Together, these timing-specific, response-specific, and cell-specific properties constitute a set of temporal-cellular coincident ‘fingerprint markers’ that unequivocally establish excitatory connectivity of the red neuronal pool in pons to that in medulla—in a manner analogous to fingerprint matching. The methods of stimulation and suppression of neuronal activity may vary depending on the cellular properties of the neuronal populations of interest but the set of temporal-cellular markers should be chosen to uniquely verify neuronal connectivity. Inhibitory connectivity between neuronal pools whose discharge patterns are anti-synchronized may also be discerned in a similar fashion. For the assay as depicted above, possible functional connectivity from medullary pools to corresponding pontine pools cannot be ruled out and must be tested separately. In this study, temporal-cellular coincident fingerprint markers are provided by a set of neuronal characteristics including post-I phase specificity, hypoxia-induced short-term potentiation, and critical dependence on NMDAR activity of the pontine pneumotaxic mechanism as manifested at the systems, network, and cellular levels, as demonstrated in Figs. 2-6 below. Note that the long-range functional connectivity so determined could be either monosynaptic or oligosynaptic, hence additional tests (such as demonstrated in Fig. 3E) are necessary to address this issue.
