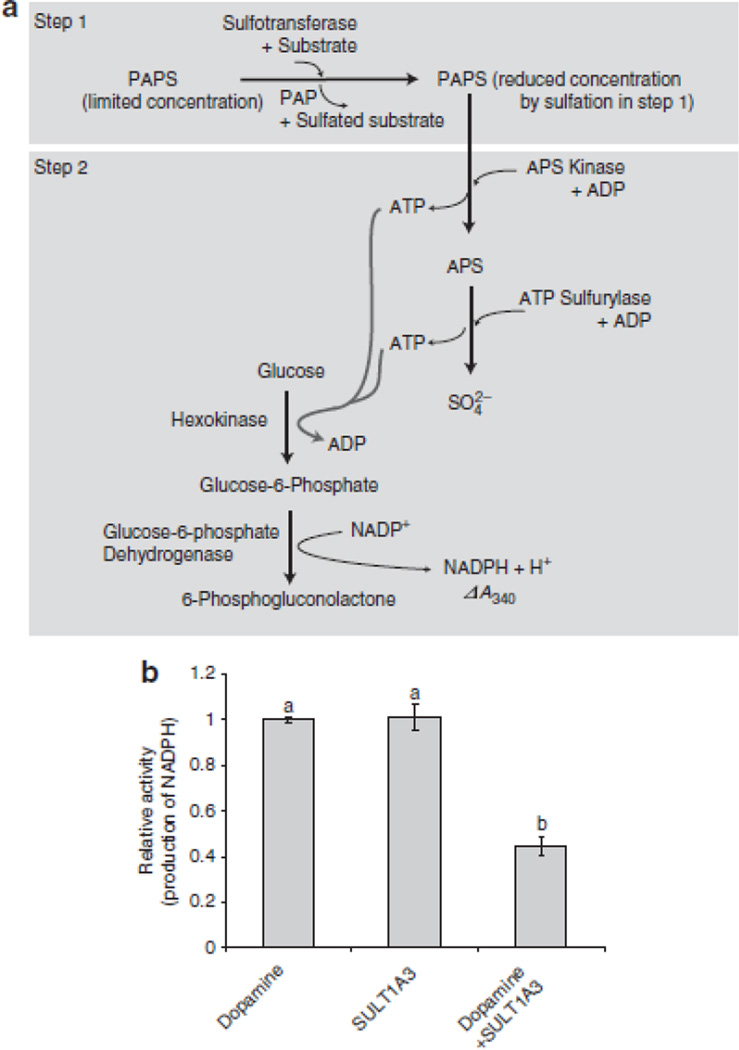
Figure 1. Development of a sulfotransferase activity assay.
(A) In the first step, a sulfotransferase, a substrate, and PAPS are incubated under conditions that allow for the production of a sulfated substrate. In the next step, APS kinase and ATP sulfurylase catalyze reverse reactions to produce APS and ATP from the PAPS remaining from the first step. If PAPS is limiting, less APS and ATP will be produced. A subsequent reaction catalyzed by hexokinase and glucose-6-phosphate dehydrogenase utilizes the ATP and glucose to produce the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) and 6-phosphogluconolactone. NADPH production can be specifically monitored at 340 nm using a spectrophotometer. In this assay system, two moles of NADPH are produced for each mole of PAPS turned over by APS kinase26. Given an excess of PAPS and all other components in each step, the reaction rate will proceed in the absence of a sulfotransferase or its substrate (the sulfuryl-group receiver). If, however, an active sulfotransferase and substrate are incubated together prior to the addition of APS kinase under limited concentration of PAPS, NADPH production is decreased compared to that detected in reactions lacking a sulfotransferase or its substrate. This decrease reflects the presence of sulfotransferase enzymatic activity. Specific conditions are described in Methods. (B) Validation of the enzyme coupling sulfotransferase assay system using dopamine sulfotransferase, SULT1A3 (25 units), and its substrate, dopamine (60 µM). NADPH production was measured at 340 nm using a spectrophotometer. Bars indicate the relative average value obtained for ΔA340/min ± standard deviation in three independent experiments. Different letters on the top of each bar indicate statistically significant differences between treatments [(one way ANOVA analysis (Holm-Sidak test, P<0.05)].